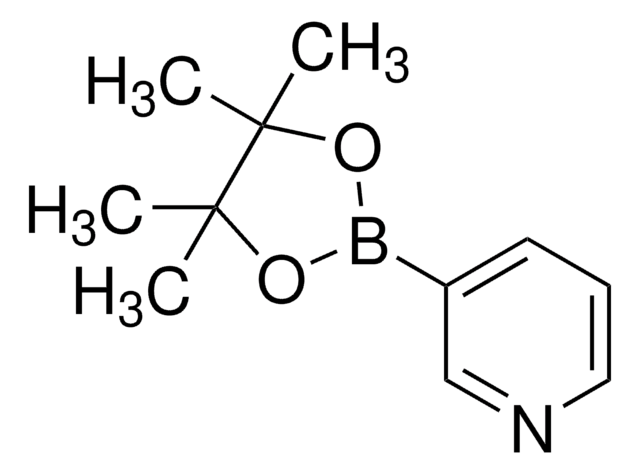
578401
3-Cyanophenylboronic acid pinacol ester
97%
Synonym(s):
3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H16BNO2
CAS Number:
Molecular Weight:
229.08
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
78-82 °C (lit.)
functional group
nitrile
SMILES string
CC1(C)OB(OC1(C)C)c2cccc(c2)C#N
InChI
1S/C13H16BNO2/c1-12(2)13(3,4)17-14(16-12)11-7-5-6-10(8-11)9-15/h5-8H,1-4H3
InChI key
FIGQEPXOSAFKTA-UHFFFAOYSA-N
Related Categories
Application
3-Cyanophenylboronic acid pinacol ester can be used as a reactant to synthesize:
- 3-Cyano aryl/ heteroaryl derivatives by forming a C−C bond via palladium-catalyzed Suzuki-Miyaura reaction.
- 3-(Phenylamino)benzonitrile by copper-catalyzed Chan-Evans-Lam amination reaction.
- 3-(Trifluoromethyl)benzonitrile using potassium (trifluoromethyl)trimethoxyborate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chan-Evans-Lam amination of boronic acid pinacol (BPin) esters: overcoming the aryl amine problem
Vantourout JC, et al.
The Journal of Organic Chemistry, 81(9), 3942-3950 (2016)
Oxidative Trifluoromethylation of Arylboronates with Shelf-Stable Potassium (Trifluoromethyl) trimethoxyborate
Khan BA, et al.
Chemistry?A European Journal , 18(6), 1577-1581 (2012)
Speciation control during Suzuki-Miyaura cross-coupling of haloaryl and haloalkenyl MIDA boronic esters
Fyfe JWB, et al.
Chemistry?A European Journal , 21(24), 8951-8964 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-5,10-diboronic acid bis(pinacol) ester 95%](/deepweb/assets/sigmaaldrich/product/structures/396/334/bb0914db-5c9a-4565-ba76-30dd4bc4ba87/640/bb0914db-5c9a-4565-ba76-30dd4bc4ba87.png)