All Photos(1)
About This Item
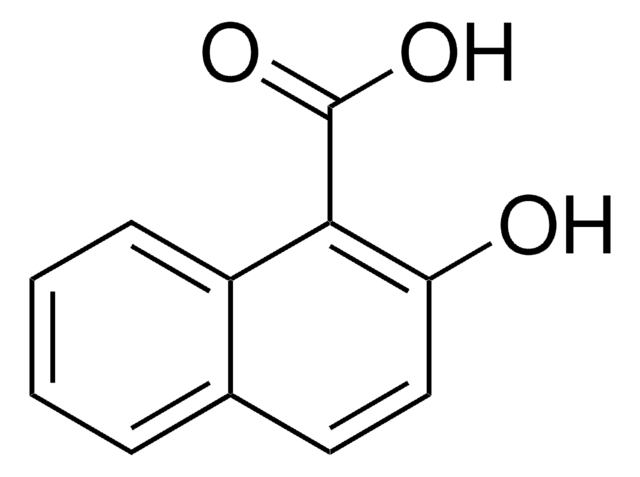
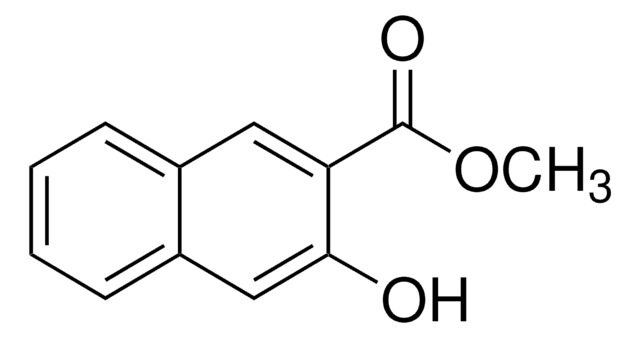
Linear Formula:
HOC10H6CO2CH3
CAS Number:
Molecular Weight:
202.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
76-80 °C (lit.)
functional group
ester
SMILES string
COC(=O)c1ccc2ccccc2c1O
InChI
1S/C12H10O3/c1-15-12(14)10-7-6-8-4-2-3-5-9(8)11(10)13/h2-7,13H,1H3
InChI key
HMIBDRSTVGFJPB-UHFFFAOYSA-N
General description
The asymmetric unit of the crystal of methyl 1-hydroxy-2-naphthoate contains two independent planar molecules that exhibit intramolecular hydrogen bonds. Methyl 1-hydroxy-2-naphthoate can be prepared from 1-hydroxy-2-naphthoic acid, via esterification.
Application
Methyl 1-hydroxy-2-naphthoate may be used as a starting reagent for the synthesis of axially chiral benzimidazole derivatives.
It may also be employed in the synthesis of the following aza-mollugin derivatives:
It may also be employed in the synthesis of the following aza-mollugin derivatives:
- azamollugin
- 2-desmethyl-azamollugin
- 2,2-didesmethyl-azamollugin
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hitomi Nishino et al.
Bioorganic & medicinal chemistry letters, 26(2), 524-525 (2015-12-19)
Oxomollugin (2) is a degradation product of mollugin (1) and a potent inhibitor of NO-production including nuclear factor kappa B signals. In our endeavor to develop a potent anti-inflammatory compound, we synthesized several aza-derivatives of oxomollugin (2) and evaluated their
Feijun Wang et al.
Beilstein journal of organic chemistry, 8, 726-731 (2012-09-28)
Axially chiral oxazoline-carbene ligands with an N-naphthyl framework were successfully prepared, and their coordination behavior with AuCl·SMe(2) was also investigated, affording the corresponding Au(I) complexes in moderate to high yields.
Methyl 1-hydroxy-2-naphthoate.
Jin LF and Xiao FP.
Acta Crystallographica Section E, Structure Reports Online, 61(5), o1520-o1522 (2005)
Effects of electronic structures on the excited-state intramolecular proton transfer of 1-hydroxy-2-acetonaphthone and related compounds.
Tobita S, et al.
The Journal of Physical Chemistry A, 102(27), 5206-5214 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service