All Photos(1)
About This Item
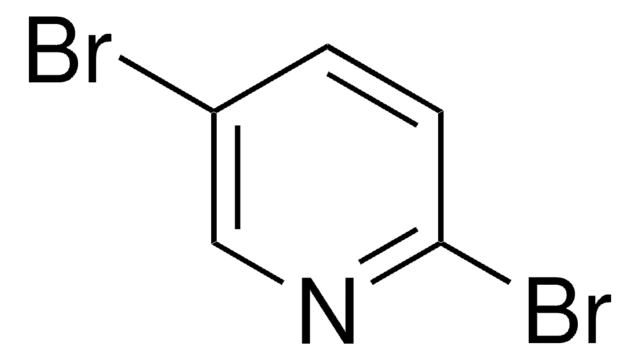
Empirical Formula (Hill Notation):
C5H5IN2
CAS Number:
Molecular Weight:
220.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
128-131 °C (lit.)
functional group
iodo
SMILES string
Nc1ccc(I)cn1
InChI
1S/C5H5IN2/c6-4-1-2-5(7)8-3-4/h1-3H,(H2,7,8)
InChI key
IVILGUFRMDBUEQ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Transition metal halide salts of 2-amino-5-substituted-pyridines: Synthesis, crystal structure and magnetic properties of two polymorphs of (5-IAP)2CuCl4 [5-IAP= 2-amino-5-iodopyridinium].
Giantsidis J, et al.
Journal of Coordination Chemistry, 55(7), 795-803 (2002)
Zhi-Ping Zhuang et al.
Journal of medicinal chemistry, 46(2), 237-243 (2003-01-10)
A series of novel beta-amyloid (A beta) aggregate-specific ligands, 2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyridine, 16(IMPY), and its related derivatives were prepared. An in vitro binding study with preformed A beta aggregates showed that 16(IMPY) and its bromo derivative competed with binding of 2-(4'-dimethylaminophenyl)-6-iodobenzothiazole, [125I]7(TZDM)
Substituted 2-Sulfonamido-5-aminopyridines. II.
Caldwell WT, et al.
Journal of the American Chemical Society, 66(9), 1479-1484 (1944)
N Sundaraganesan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 67(3-4), 830-836 (2006-10-05)
The Fourier transform Raman and Fourier transform infrared spectra of 2-amino-5-iodopyridine were recorded in the solid phase. The equilibrium geometry, harmonic vibrational frequencies, infrared intensities and Raman scattering activities were calculated by HF and DFT (B3LYP) methods with the 6-31G(d,p)
R J Bochis et al.
Journal of medicinal chemistry, 21(2), 235-237 (1978-02-01)
A series of methyl imidazo-[11,2-a]pyridine-2-carbamates was synthesized for anthelmintic testing. The preparation of this class of compounds was simplified by utilization of a novel one-step condensation of the appropriately substituted 2-aminopyridine and methyl chloroacetylcarbamate. The most potent compound, methyl 6-(phenylsulfinyl)-imidazo[1,2-a]pyridine-2-carbamate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service