All Photos(2)
About This Item
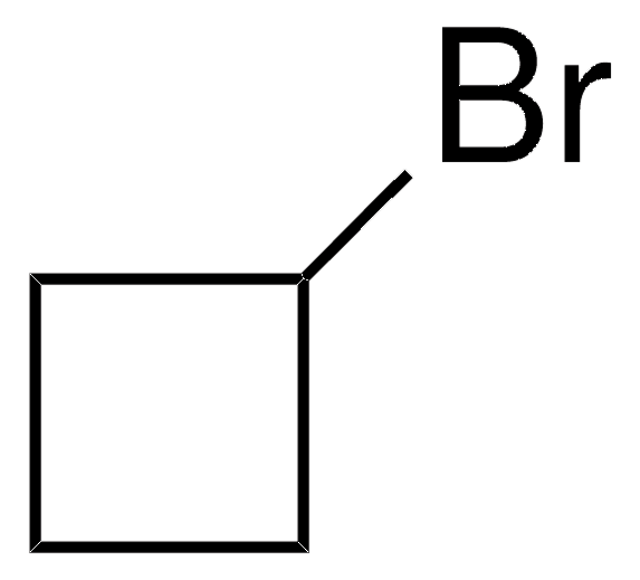
Linear Formula:
C3H5CH2CN
CAS Number:
Molecular Weight:
81.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.4235 (lit.)
bp
142-144 °C (lit.)
density
0.878 g/mL at 25 °C (lit.)
functional group
nitrile
SMILES string
N#CCC1CC1
InChI
1S/C5H7N/c6-4-3-5-1-2-5/h5H,1-3H2
InChI key
FAUQRRGKJKMEIW-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
111.0 °F - closed cup
Flash Point(C)
43.89 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Phyllis A Leber et al.
Molecules (Basel, Switzerland), 19(2), 1527-1543 (2014-01-30)
The title compound 1-exo (with minor amounts of its C8 epimer 1-endo) was prepared by Wolff-Kishner reduction of the cycloadduct of 1,3-cyclohexadiene and cyclopropylketene. The [1,3]-migration product 2-endo was synthesized by efficient selective cyclopropanation of endo-5-vinylbicyclo[2.2.2]oct-2-ene at the exocyclic π-bond.
The Synthesis of Methyl Cyclopropylacetate by Palladium (II) Acetate Catalysed Reaction Of Diazomethane with Vinyl Group.
Basnak I, et al.
Synthetic Communications, 22(5), 773-782 (1992)
Jonathan D Rosen et al.
Tetrahedron letters, 50(7), 785-789 (2010-02-18)
An optimized total synthesis of the 2-amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core structure of a new fluoroquinolone-like class of antibacterial agents is described. This synthesis is highlighted by a nearly quantitative ring-closing reaction to form the pyrido[1,2-c]pyrimidine core. This bicyclic ring system serves as
Charles E Mowbray et al.
Journal of medicinal chemistry, 58(24), 9615-9624 (2015-11-17)
Visceral leishmaniasis is a severe parasitic disease that is one of the most neglected tropical diseases. Treatment options are limited, and there is an urgent need for new therapeutic agents. Following an HTS campaign and hit optimization, a novel series
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service