50640
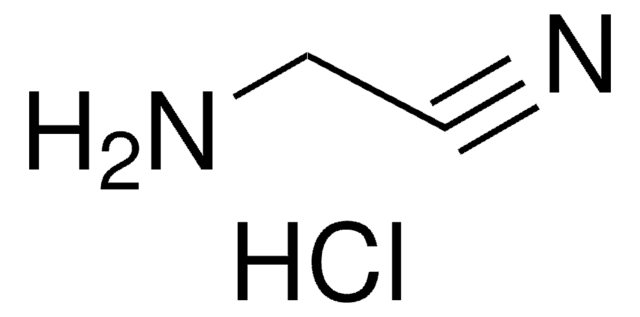
Glycolic acid nitrile solution
~70% in H2O
Synonym(s):
Formaldehyde cyanohydrin, Hydroxyacetonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2CN
CAS Number:
Molecular Weight:
57.05
Beilstein:
605328
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
contains
~0.5% phosphoric acid as stabilizer
Quality Level
concentration
~70% in H2O
refractive index
n20/D 1.389
density
1.076 g/mL at 20 °C
functional group
hydroxyl
SMILES string
OCC#N
InChI
1S/C2H3NO/c3-1-2-4/h4H,2H2
InChI key
LTYRAPJYLUPLCI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The product is a 70% solution of glycolic acid nitrile in water. Glycolic acid nitrile, also known as hydroxyacetonitrile is the simplest cyanohydrin. The microwave spectra of hydroxyacetonitrile have been recorded in gas-phase and its rotational constants have been calculated. Ice containing formaldehyde and hydrogen cyanide react under astrophysical-like conditions to form hydroxyacetonitrile.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Formation of hydroxyacetonitrile (HOCH2CN) and polyoxymethylene (POM)-derivatives in comets from formaldehyde (CH2O) and hydrogen cyanide (HCN) activated by water.
Danger G, et al.
Physical Chemistry Chemical Physics, 16(8), 3360-3370 (2014)
Microwave and Quantum-Chemical Study of Conformational Properties and Intramolecular Hydrogen Bonding of 2-Hydroxy-3-Butynenitrile (HC=CCH(OH)C=N).
M?llendal H, et al.
The Journal of Physical Chemistry A, 119(4), 634-640 (2015)
Hydroxyacetonitrile (HOCH2CN) formation in astrophysical conditions. competition with the aminomethanol, a glycine precursor.
Danger G, et al.
The Astrophysical Journal, 756(1), 11-11 (2012)
Rotational isomerism and barriers to internal rotation in hydroxyacetonitrile from microwave spectroscopy.
Cazzoli G, et al.
J. Chem. Soc., Faraday II, 69, 569-578 (1973)
G Arrhenius et al.
The Journal of organic chemistry, 62(16), 5522-5525 (1997-08-08)
A study of glycolonitrile polymerization has led to the isolation and characterization of two 2,5-dihydro-4-aminooxazoles, 4 and 5. Previous reports have misassigned these structures as s-triazines or pyrimidines. X-ray diffraction analysis of crystals of 4 and an acetylated oxazole derivative
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service