484903
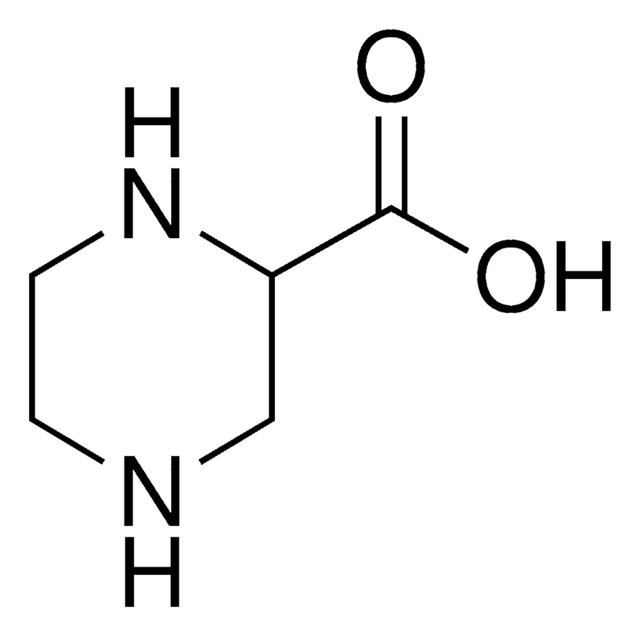
Piperazine-2-carboxylic acid dihydrochloride
98%
Synonym(s):
(±)-Piperazine-2-carboxylic acid dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H10N2O2 · 2HCl
CAS Number:
Molecular Weight:
203.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
265 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
Cl[H].Cl[H].OC(=O)C1CNCCN1
InChI
1S/C5H10N2O2.2ClH/c8-5(9)4-3-6-1-2-7-4;;/h4,6-7H,1-3H2,(H,8,9);2*1H
InChI key
WNSDZBQLMGKPQS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maryam Hashemi et al.
Materials science & engineering. C, Materials for biological applications, 61, 791-800 (2016-02-04)
Poly-(amidoamine) (PAMAM) and poly-(propylenimine) (PPI) are the two most widely investigated dendrimers for drug and gene delivery. In order to enhance DNA transfection activity of these dendrimers, generation 3 and 4 PAMAM and generation 4 and 5 PPI were modified
Zahra Salmasi et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 76-88 (2015-07-26)
Branched polyethylenimine (PEI) is extensively used as a polycationic non-viral vector for gene delivery. Polyplexes formed with PEI are believed to be released from endocytotic vesicles by the osmotic burst mechanism in the rate-limiting step in transfection. Increasing the buffering
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service