483346
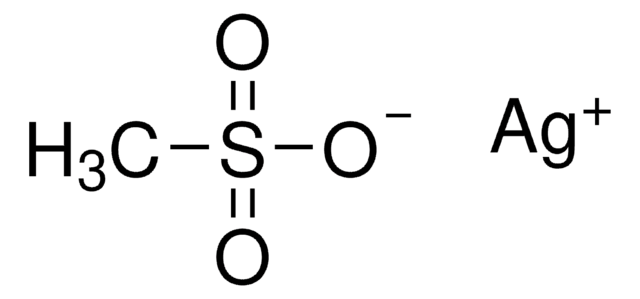
Silver trifluoromethanesulfonate
≥99.95% trace metals basis
Synonym(s):
AgOTf, Silver triflate, Trifluoromethanesulfonic acid silver salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CF3SO3Ag
CAS Number:
Molecular Weight:
256.94
Beilstein:
3598402
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.95% trace metals basis
reaction suitability
core: silver
reagent type: catalyst
mp
286 °C (lit.)
SMILES string
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
QRUBYZBWAOOHSV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Silver trifluoromethanesulfonate (AgOTf ) is a reactive triflating agent, which converts alkyl, acyl and sulfonyl halides to corresponding triflate species.
- It is a highly suitable electrophile to initiate acetylenic oxy-Cope rearrangement of substituted 5-hexen-1-yn-3-ols to synthesize corresponding α,δ-diethylenic aldehydes.
- It can also be used in the diastereoselective cyclization of amino ketenes where the diastereoselectivity depends on Ag(I) concentration.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Trifluoromethanesulfonic?Carboxylic Anhydrides, Highly Active Acylating Agents.
Effenberger F and Epple G
Angewandte Chemie (International Edition in English), 11(4), 299-300 (1972)
Perfluoroalkanesulfonic esters: methods of preparation and applications in organic chemistry.
Stang P J, et al.
Synthesis, 1982(02), 85-126 (1982)
Silver mediated acetylenic oxy cope rearrangement.
Bluthe N, et al.
Tetrahedron, 42(5), 1333-1344 (1986)
Asymmetric synthesis via electrophile-mediated cyclisations.
Fox D N and Gallagher T
Tetrahedron, 46(13-14), 4697-4710 (1990)
Lingyong Jiang et al.
Organic & biomolecular chemistry, 10(40), 8102-8107 (2012-09-06)
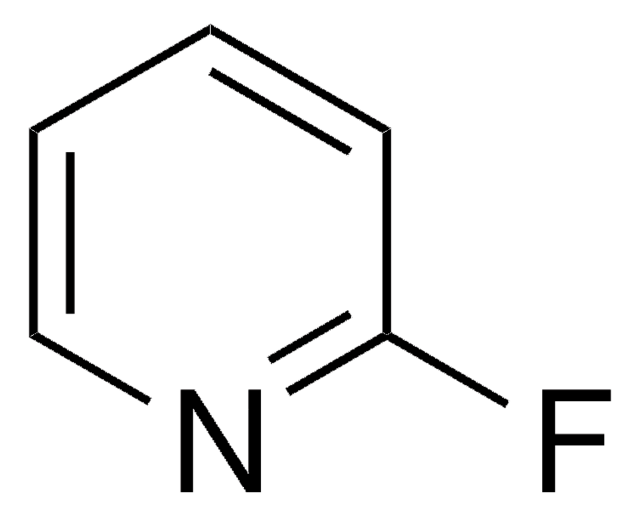
A silver triflate-catalyzed tandem reaction of N'-(2-alkynylbenzylidene)hydrazide with pyridyne is presented. Different outcomes are obtained, depending on the pyridynes utilized in the transformation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)

