482099
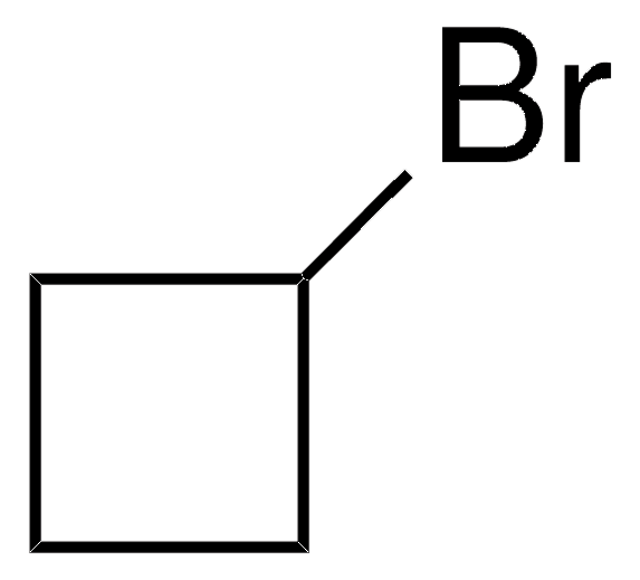
Cyclopropanemethanol
≥99.5%
Synonym(s):
(Hydroxymethyl)cyclopropane, CPMO, Cyclopropyl carbinol, Cyclopropylmethanol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C3H5CH2OH
CAS Number:
Molecular Weight:
72.11
Beilstein:
1846846
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.5%
refractive index
n20/D 1.431 (lit.)
bp
123-124 °C/738 mmHg (lit.)
density
0.89 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OCC1CC1
InChI
1S/C4H8O/c5-3-4-1-2-4/h4-5H,1-3H2
InChI key
GUDMZGLFZNLYEY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cyclopropanemethanol (Cyclopropyl carbinol, CPMO), a cycloalkanemethanol, is an anaesthetic. The coupling reaction of cyclopropanemethanol with alkynes to form substituted allylic alcohols has been reported. The microwave spectrum of CPMO has been recorded. Its rotational constants and dipole moment have been determined.
Application
Cyclopropanemethanol may be used in the preparation of:
- cyclopropanecarbaldehyde
- cyclopropylmethylsulfonate
- dibenzyl cyclopropylmethyl phosphate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
95.0 °F - closed cup
Flash Point(C)
35 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The microwave spectrum, dipole moment, and structure of cyclopropyl carbinol.
Bhaumik A, et al.
Canadian Journal of Chemistry, 48(19) , 2949-2954 (1970)
Cyclopropylmethyl dihydrogen phosphate. Preparation and use in the phosphorylation of nucleosides.
Schoffstall AM.
The Journal of Organic Chemistry, 40(23), 3444-3445 (1975)
Nickel-Catalyzed Redox-Economical Coupling of Alcohols and Alkynes to Form Allylic Alcohols.
Nakai K, et al.
Journal of the American Chemical Society, 136(22), 7797-7800 (2014)
Galvanic Deposition of Au on Paperlike Cu Fiber for High-Efficiency, Low-Temperature Gas-Phase Oxidation of Alcohols.
Zhao G, et al.
ChemCatChem, 3(10), 1629-1636 (2011)
Surbhi Soni et al.
Chirality, 30(1), 85-94 (2017-10-25)
A profoundly time-efficient chemoenzymatic method for the synthesis of (S)-3-(4-chlorophenoxy)propan-1,2-diol and (S)-1-chloro-3-(2,5-dichlorophenoxy)propan-2-ol, two important pharmaceutical intermediates, was successfully developed using Pseudomonas fluorescens lipase (PFL). Kinetic resolution was successfully achieved using vinyl acetate as acylating agent, toluene/hexane as solvent, and reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service