446424
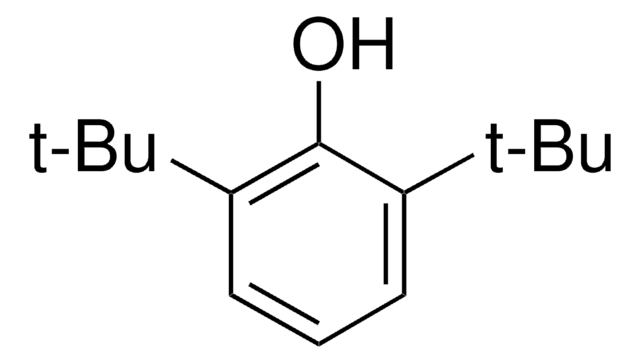
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol
97%
Synonym(s):
3,5-Di-tert-butyl-4-hydroxyphenylmethanol, 4-Hydroxymethyl-2,6-di-tert-butylphenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H2[C(CH3)3]2CH2OH
CAS Number:
Molecular Weight:
236.35
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
139-141 °C (lit.)
functional group
hydroxyl
SMILES string
CC(C)(C)c1cc(CO)cc(c1O)C(C)(C)C
InChI
1S/C15H24O2/c1-14(2,3)11-7-10(9-16)8-12(13(11)17)15(4,5)6/h7-8,16-17H,9H2,1-6H3
InChI key
HNURKXXMYARGAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol can be used as a reactant to synthesize:
- 2,6-di-tert-butyl-4-(dodecylselanylmethyl)phenol and bis(3,5-di-tert-butyl-4-hydroxybenzyl) selenide by reacting with dodecaneselenolate and sodium selenide.
- Monomeric antioxidant by reacting with imidazole and N-[4-(chlorocarbonyl)phenyl]maleimide.
- Sulfur-containing butylated hydroxytoluene derivatives by reacting with aryl/alky dithiols.
- 3,5-Di-tert-butyl-4-hydroxybenzaldehyde by oxidation reaction using stabilized IBX.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Melt-grafting of maleimides having hindered phenol group onto polypropylene.
Kim TH and Lee N.
Bull. Korean Chem. Soc., 24(12), 1809-1813 (2003)
X Guan et al.
Carcinogenesis, 16(10), 2575-2582 (1995-10-01)
The mouse pneumotoxicant and lung and liver tumor promoter butylated hydroxytoluene (BHT) was examined for its effects on gap junctional intercellular communication (GJIC) in mouse lung epithelial (C10) and rat liver epithelial (WB-F344) cell lines. GJIC, as measured by fluorescent
K D Munkres
Mechanisms of ageing and development, 5(3), 163-169 (1976-05-01)
Clonal growth rate and cellular viability of an inositol-less mutant of Neurospora crassa decline rapidly during deprivation of dietary inositol. Dietary antioxidants, either nordihydroguaiaretic acid, vitamin E or 3,5-ditert.-butyl-4-hydroxybenzyl alcohol, protected cells and clones of the mutant from death and
The antioxidant activity of 3, 5-di-tert-butyl-4-hydroxybenzyl derivatives.
Kim DH and Kummerow FA.
Journal of the American Chemical Society, 39(3), 150-155 (1962)
Synthesis of new polymeric antioxidants.
Oh DR, et al.
Bull. Korean Chem. Soc., 22(6), 629-632 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service