444286
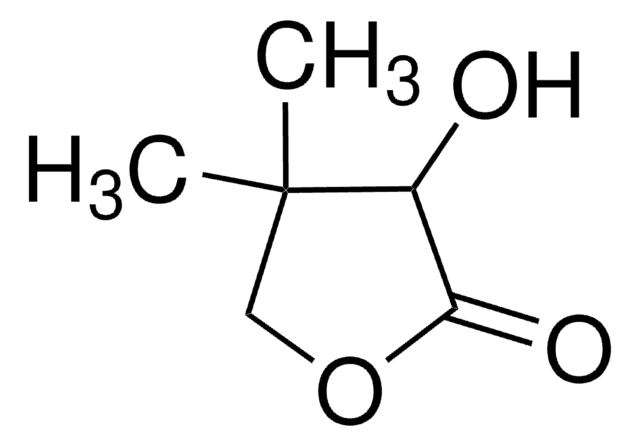
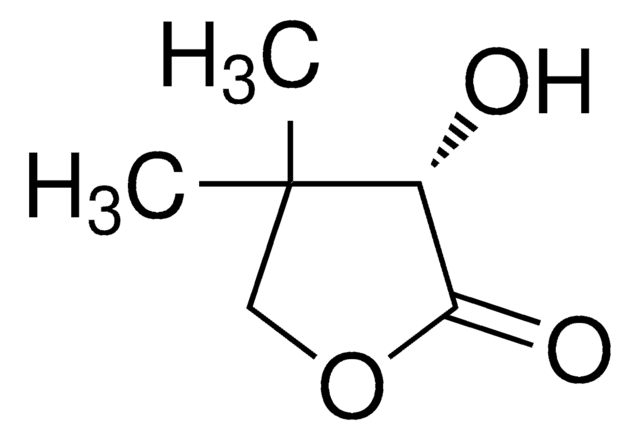
(R)-(+)-α-Hydroxy-γ-butyrolactone
95%, optical purity ee: 98% (GLC)
Synonym(s):
(R)-4,5-Dihydro-3-hydroxy-2(3H)-furanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O3
CAS Number:
Molecular Weight:
102.09
Beilstein:
80588
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
optical activity
[α]23/D +66°, c = 1.15 in chloroform
optical purity
ee: 98% (GLC)
refractive index
n20/D 1.467 (lit.)
bp
133 °C/10 mmHg (lit.)
density
1.309 g/mL at 25 °C (lit.)
SMILES string
O[C@@H]1CCOC1=O
InChI
1S/C4H6O3/c5-3-1-2-7-4(3)6/h3,5H,1-2H2/t3-/m1/s1
InChI key
FWIBCWKHNZBDLS-GSVOUGTGSA-N
Application
(R)-(+)-α-Hydroxy-γ-butyrolactone can be used as a starting material to synthesize:
- δ-Azaproline by reacting with benzyloxycarbonyl aminophthalimide via Mitsunobu reactions.
- Homochiral (R)-2,4-dihydroxybutyramide seco-pseudonucleoside reagents.
- Botryolide B via esterification and ring-closing metathesis reaction.
- Pregnane derivatives containing γ-butyrolactones as potential glucocorticoid agonists.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel glucocorticoid antedrugs possessing a 21-(?-lactone) ring.
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 6, 831-839 (2002)
Concise total synthesis of botryolide B
Mohapatra DK, et al.
Royal Society of Chemistry Advances, 4(16), 8335-8340 (2014)
Novel glucocorticoid antedrugs possessing a 21-(γ-lactone) ring
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 4(6), 831-839 (2002)
Xiaohui Gou et al.
Frontiers in physiology, 11, 686-686 (2020-07-17)
Dentin sialoprotein (DSP), the NH2-terminal fragment of dentin sialophosphoprotein (DSPP), is essential for dentin formation and further processed into small fragments inside the odontoblasts. Gelatinases, including matrix metalloproteinases 9 (MMP9) and MMP2, were able to cleave DSP(P) in tooth structures.
Natalia N Dioubankova et al.
Organic letters, 4(26), 4607-4610 (2002-12-20)
[reaction: see text] Two series of seco-pseudonucleoside synthons were synthesized from (R)-(+)-alpha-hydroxy-gamma-butyrolactone and (R)-(-)-pantolactone by aminolysis, side-chain protection, dimethoxytritylation, and phosphitylation or solid-phase attachment. The phosphoramidites and solid supports were used in automated DNA synthesis to prepare oligonucleotides modified with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service