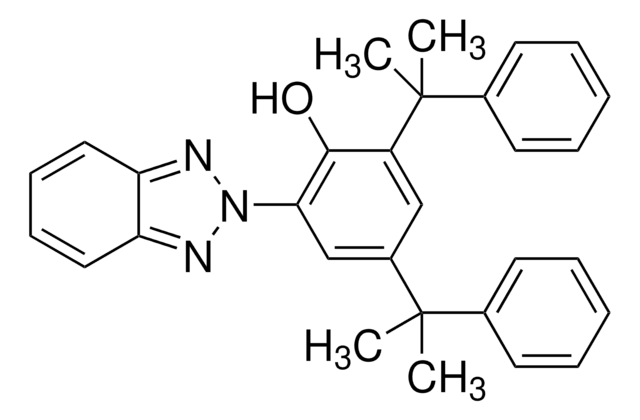
413437
2-[3-(2H-Benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate
99%
Synonym(s):
2-[2-Hydroxy-5-[2-(methacryloyloxy)ethyl]phenyl]-2H-benzotriazole
About This Item
Recommended Products
Quality Level
Assay
99%
form
solid
mp
96-98 °C (lit.)
SMILES string
CC(=C)C(=O)OCCc1ccc(O)c(c1)-n2nc3ccccc3n2
InChI
1S/C18H17N3O3/c1-12(2)18(23)24-10-9-13-7-8-17(22)16(11-13)21-19-14-5-3-4-6-15(14)20-21/h3-8,11,22H,1,9-10H2,2H3
InChI key
VCYCUECVHJJFIQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To prepare a polymer material by copolymerization with 2-hydroxy-4-acryloyloxybenzophenone (HABP). The synthesized polymer can be applied to cotton fabric for UV protection.
- In the preparation of ultraviolet (UV) protective textiles. It is grafted onto the fabric via polymerization to create a UV-protective coating.
- As a monomer in synthesizing fluoroalkyl end-capped oligomers with good surface-active properties.
- It is also used as a UV absorber (UVAs) in intraocular lenses.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2,2′-Methylenebis[6-(2H-benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol] 99%](/deepweb/assets/sigmaaldrich/product/structures/236/824/ce89085c-b9e1-4ea0-8157-44b6f9466ed6/640/ce89085c-b9e1-4ea0-8157-44b6f9466ed6.png)