412562
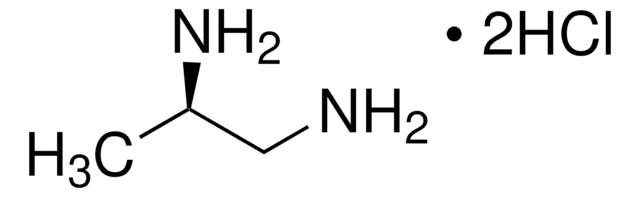
(S)-(−)-1,2-Diaminopropane dihydrochloride
99%
Synonym(s):
(S)-(−)-Propylenediamine dihydrochloride, (S)-1,2-Propanediamine dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(NH2)CH2NH2·2HCl
CAS Number:
Molecular Weight:
147.05
Beilstein:
5740936
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]22/D −4°, c = 20 in H2O
mp
227-229 °C (lit.)
functional group
amine
SMILES string
Cl.Cl.C[C@H](N)CN
InChI
1S/C3H10N2.2ClH/c1-3(5)2-4;;/h3H,2,4-5H2,1H3;2*1H/t3-;;/m0../s1
InChI key
AEIAMRMQKCPGJR-QTNFYWBSSA-N
Related Categories
Application
(S)-(-)-1,2-Diaminopropane dihydrochloride may be used in the preparation of following chiral imidazoline derivatives, which show moderate α-adrenergic blocking activity:
- (S)-(-)-4-methyl-2-(1-naphthylmethyl)imidazoline hydrochloride
- (S)-(-)-2-benzyl-4-methylimidazoline picrate
- (S )-(-)-2-[(2,6-dichlorophenyl)imino]-4-methylimidazolidine hydrochloride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Optically active derivatives of imidazolines: a-Adrenergic blocking properties.
Hsu FL, et al.
Journal of Medicinal Chemistry, 23(11), 1232-1235 (1980)
Stereochemical studies of adrenergic drugs. Optically active derivatives of imidazolines.
Miller DD, et al.
Journal of Medicinal Chemistry, 19(12), 1382-1384 (1976)
Lorin A Thompson et al.
Bioorganic & medicinal chemistry letters, 21(22), 6909-6915 (2011-10-07)
The synthesis, evaluation, and structure-activity relationships of a set of related constrained diaminopropane inhibitors of BACE-1 are described. The full in vivo profile of an optimized inhibitor in both normal and P-gp deficient mice is compared with data generated in
Keisuke Maruyoshi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(7), 1618-1626 (2009-01-09)
Endogenous polyamines, represented by putrescine, spermidine, and spermine, are known to exert their physiological functions by interacting with polyanionic biomolecules such as DNA, RNA, adenosine triphosphate (ATP), and phospholipids. Very few examples of conformation analysis have been reported for these
S A M Fathi et al.
Journal of hazardous materials, 164(1), 133-137 (2008-09-10)
Bis(5-bromo-2-hydroxybenzaldehyde)-1,2-propanediimine is synthesized by the reaction of 5-bromo-2-hydroxybenzaldehyde and 1,2-diaminopropane in ethanol. This ligand is used as a modifier of octadecyl silica disks for preconcentration of trace amounts of copper(II) ions, followed by nitric acid elution and flame atomic absorption
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service