All Photos(1)
About This Item
Empirical Formula (Hill Notation):
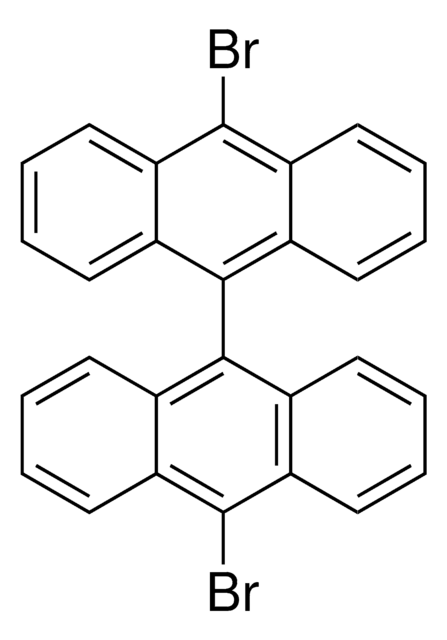
C16H9Br
CAS Number:
Molecular Weight:
281.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
powder
mp
102-105 °C (lit.)
functional group
bromo
SMILES string
Brc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C16H9Br/c17-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9H
InChI key
HYGLETVERPVXOS-UHFFFAOYSA-N
General description
1-Bromopyrene, a polycyclic aromatic hydrocarbon (PAH), is a mono bromo substituted pyrene derivative. Its synthesis has been reported. Its gas phase UV-absorption spectra in the UV wavelength range at elevated temperature has been studied. Electron affinitiy (EA) of 1-bromopyrene has been investigated using electron attachment desorption chemical ionization mass spectrometry (DCI-MS) and triple quadrupole tandem mass spectrometry. It participates in the synthesis of novel ruthenium (II) bipyridine or terpyridine complexes bearing pyrene moiety. The reaction of 1-bromopyrene cation radical with water in acetonitrile has been analyzed using the electron transfer stopped-flow (ETSF) method.
Application
1-Bromopyrene is suitable reagent used in the comparative study of effect of substituents of some pyrene derivatives in inducing phototoxicity, DNA damage and repair in human skin keratinocytes and light-induced lipid peroxidation in methanol. It is suitable reagent used in the study to investigate the UV photon-assisted thermal decomposition of PAHs at elevated temperature.
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe. It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.
It may be used in the synthesis of the following:
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe. It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.
It may be used in the synthesis of the following:
- 2-methyl-4-pyren-1-yl-but-3-yn-2-ol
- 1-ethynylpyrene
- silsesquioxane (SSQ) based hybrid
- ruthenium nanoparticles functionalized with pyrene moiety
- mono- and di-pyrenyl perfluoroalkanes
- oligo(1-bromopyrene)(OBrP) films
- dinitropyrene-derived DNA adduct
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetic analysis of the reactions of 1-substituted pyrene cation radicals with water in acetonitrile.
Oyama M and Matsui J.
Journal of Electroanalytical Chemistry, 570(1), 77-82 (2004)
Jillian L Goldfarb et al.
The Journal of chemical thermodynamics, 40(3), 460-466 (2008-03-01)
Knowledge of vapor pressures of high molar mass organics is essential to predicting their behavior in combustion systems as well as their fate and transport within the environment. This study involved polycyclic aromatic compounds (PACs) containing halogen hetero-atoms, including bromine
Gas-phase UV spectroscopy of anthracene, xanthone, pyrene, 1-bromopyrene and 1, 2, 4-trichlorobenzene at elevated temperatures.
Thony A and Rossi MJ.
Journal of Photochemistry and Photobiology A: Chemistry, 104(1), 25-33 (1997)
Syntheses of mono-and di-pyrenyl perfluoroalkanes.
Wiedenfeld D, et al.
Journal of Fluorine Chemistry, 104(2), 303-306 (2000)
M Shou et al.
Drug metabolism and disposition: the biological fate of chemicals, 16(2), 173-183 (1988-03-01)
Due to the symmetrical property of pyrene (Py), trans-dihydrodiols formed at 4,5- and 9,10-positions are identical, as are the monohydroxylated products (phenols) formed at C1, C3, C6, and C8 positions. With a bromo substituent at C1 position of Py, 1-bromopyrene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service