All Photos(1)
About This Item
Empirical Formula (Hill Notation):
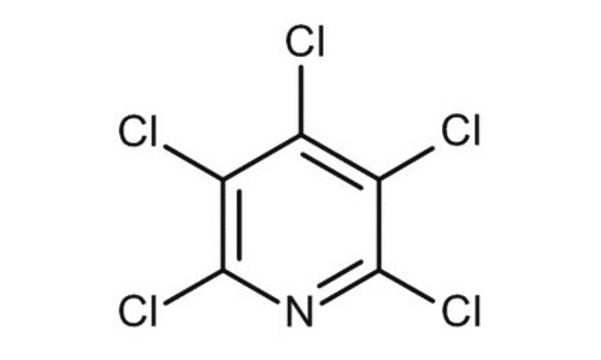
C5H2Cl3N
CAS Number:
Molecular Weight:
182.44
Beilstein:
119384
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
219 °C (lit.)
mp
46-50 °C (lit.)
functional group
chloro
SMILES string
Clc1cnc(Cl)c(Cl)c1
InChI
1S/C5H2Cl3N/c6-3-1-4(7)5(8)9-2-3/h1-2H
InChI key
CNLIIAKAAMFCJG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Separation and migration behavior of 2,3,5-trichloropyridine has been studied by micellar electrokinetic chromatography. Crystal structure of 2,3,5-trichloropyridine has been reported. Molecules of 2,3,5-trichloropyridine in crystal are stacked along the short a axis and forms a layer structure. 2,3,5-Trichloropyridine is reported to undergo nucleophilic displacement reaction in ionic liquid to afford the corresponding 2-aryloxylpropionate.
Application
2,3,5-Trichloropyridine may be used in the synthesis of 3,5-dichloro-2-arylpyridines via palladium acetate-catalyzed ligand-free Suzuki reaction with arylboronic acids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C E Lin et al.
Journal of chromatography. A, 910(1), 165-171 (2001-03-27)
The separation and migration behavior of pyridine and eight chloropyridines, including three monochloropyridines, four dichloropyridines, and 2,3,5-trichloropyridine were investigated by micellar electrokinetic chromatography using either sodium dodecyl sulfate (SDS) as an anionic surfactant or SDS-Brij 35 mixed micelles. Various parameters
Huanan Hu et al.
Molecules (Basel, Switzerland), 14(9), 3153-3160 (2009-09-29)
A highly efficient palladium acetate-catalyzed ligand-free Suzuki reaction of 2,3,5-trichloropyridine with arylboronic acids in aqueous phase was developed. High yields of 3,5-dichloro-2-arylpyridines, a simple Pd source, absence of ligands, and environmentally benign as well as mild reaction conditions are important
Direct Formation of 2, 3, 5-Trichloropyridine and its Nucleophilic Displacement Reactions in Ionic Liquid.
Zhong P, et al.
Synthetic Communications, 34(23), 4301-4311 (2004)
2, 3, 5-Trichloropyridine.
Ma H-F, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(1), o311-o312 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service