All Photos(1)
About This Item
Empirical Formula (Hill Notation):
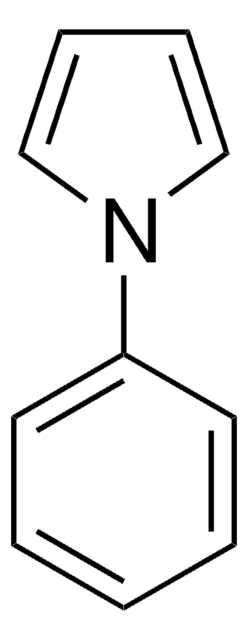
C13H25NSi
CAS Number:
Molecular Weight:
223.43
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.492 (lit.)
bp
78 °C/0.4 mmHg (lit.)
density
0.904 g/mL at 25 °C (lit.)
SMILES string
CC(C)[Si](C(C)C)(C(C)C)n1cccc1
InChI
1S/C13H25NSi/c1-11(2)15(12(3)4,13(5)6)14-9-7-8-10-14/h7-13H,1-6H3
InChI key
FBQURXLBJJNDBX-UHFFFAOYSA-N
General description
1-(Triisopropylsilyl)pyrrole (TISP), a heterocyclic building block, is a pyrrole derivative. TISP has been reported to generate pyrrolic cation radicals during cyclovoltammetric studies, via electroreduction. It participates in various electrophilic substitution reactions specifically at β-position, via reaction with various electrophilic reagents (Br+, I+,NO2+,etc).
Application
1-(Triisopropylsilyl)pyrrole may be employed as reagent in perfluoroalkylation and Vilsmeier formylation reactions. It may be used in the preparation of:
- ethyl 2-(2,4-dinitrophenylhydrazono]-3-[ 1-(triisopropylsily1)-pyrrol-2-yflpropanoate
- heterocyclic base, 3-nitropyrrole
- 3-nitropyrrole, required for the synthesis of 1 -(2′-deoxy-β-D-ribofuranosyl)-3-nitropyrrole
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
224.6 °F - closed cup
Flash Point(C)
107 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthetic Communications, 24, 2049-2049 (1994)
Reaction of pyrroles with ethyl 2-nitroso-and 2-azo-propenoates, and with ethyl cyanoformate N-oxide: a comparison of the reaction pathways.
Gilchrist TL and Lemos A.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1391-1395 (1993)
Synthesis, Structure, and Deoxyribonucleic Acid Sequencing with a Universal Nucleoside: 1-(2'-Deoxy-. beta.-D-ribofuranosyl)-3-nitropyrrole.
Bergstrom DE, et al.
Journal of the American Chemical Society, 117(4), Synthesis-Synthesis (1999)
N-(triisopropylsilyl) pyrrole. A progenitor" par excellence" of 3-substituted pyrroles.
Bray BL, et al.
The Journal of Organic Chemistry, 55(26), 6317-6328 (1990)
Tetrahedron, 49, 4015-4015 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service