All Photos(1)
About This Item
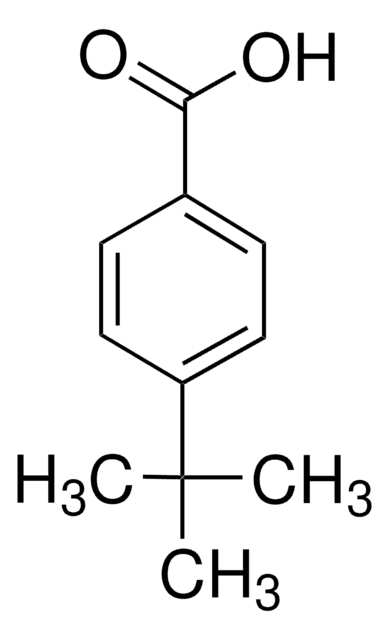
Linear Formula:
(CH3)3CC6H10CO2H
CAS Number:
Molecular Weight:
184.28
Beilstein:
2043187
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
EC Index Number:
611-163-8
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
148 °C (lit.)
functional group
carboxylic acid
SMILES string
CC(C)(C)C1CCC(CC1)C(O)=O
InChI
1S/C11H20O2/c1-11(2,3)9-6-4-8(5-7-9)10(12)13/h8-9H,4-7H2,1-3H3,(H,12,13)
InChI key
QVQKEGYITJBHRQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
delta pKa between the two stereoisomers of 4-tert-butylcyclohexanecarboxylic acid has been studied using 1H and 13C NMR. Preparation of cis- and trans-4-tert- butylcyclohexanecarboxylic acid has been reported.
Application
4-tert-Butylcyclohexanecarboxylic acid may be used in the preparation of N-((S)-3-(4-(7-chloroquinolin-4-yl)piperazin-1-yl)-1-henylpropyl)-4-tert-butylcyclohexanecarboxamide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C L Perrin et al.
Analytical chemistry, 68(13), 2127-2134 (1996-07-01)
An NMR titration method has been developed to simultaneously measure the difference in acid dissociation constants (delta pKa) of two or more compounds with high precision and accuracy. The delta pKa between the conjugate acids of the two stereoisomers of
Conformational analysis. LXXV. Methylation rates of cis-and trans-4-tert-butyl-N, N-dimethylcyclohexylamines.
Allinger NL and Graham JC.
The Journal of Organic Chemistry, 36(12), 1688-1690 (1971)
Yanqing Liu et al.
Molecules (Basel, Switzerland), 13(10), 2426-2441 (2008-10-03)
CCR5, as the major co-receptor for HIV-1 entry, is an attractive novel target for the pharmaceutical industry in the HIV-1 therapeutic area. In this study, based on the structures of maraviroc and 1,4-bis(4-(7-chloroquinolin-4-yl)piperazin-1-yl)butane-1,4-dione (1), which was identified using structure-based virtual
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service