345164
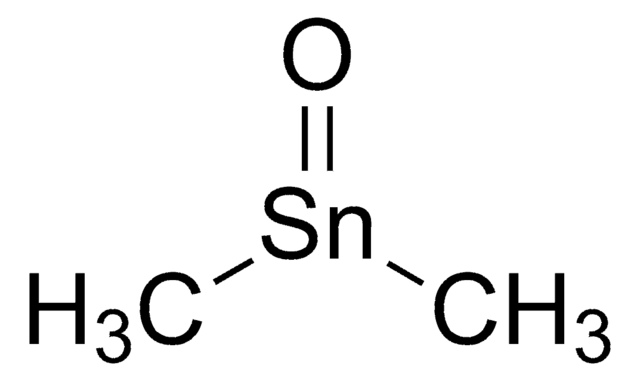
Tin(II) acetate
Synonym(s):
Stannous acetate, Tin acetate, Tin diacetate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Sn(CH3CO2)2
CAS Number:
Molecular Weight:
236.80
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
reaction suitability
core: tin
reagent type: catalyst
mp
180-182 °C (lit.)
SMILES string
CC(=O)O[SnH2]OC(C)=O
InChI
1S/2C2H4O2.Sn/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
PNOXNTGLSKTMQO-UHFFFAOYSA-L
Related Categories
General description
Tin(II) acetate can be used as a photocatalyst to produce Arylstannane(IV) Reagents.
Application
- Enhances the rate of thermal depolymerization of poly(lactic acid) fibers
- Reactant for the synthesis of Sn-Cu bimetallic nanoparticles
- used in in preparation of tin anode by organic electroplating for rechargeable thin-film batteries
- Used as tin source for preparation of high surface area tin oxide catalysts
Used to prepare tin(IV) oxide thin films by photochemical vapor deposition. With palladium(II) acetate, an efficient catalytic system for C-H activation of the methoxy group of anisole.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Junshan Li et al.
ChemSusChem, 12(7), 1451-1458 (2019-01-25)
Co-Sn solid-solution nanoparticles with Sn crystal structure and tuned metal ratios were synthesized by a facile one pot solution-based procedure involving the initial reduction of a Sn precursor followed by incorporation of Co within the Sn lattice. These nanoparticles were
Gianvito Caputo et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(12), 1635-1641 (2017-04-04)
The localized in situ formation of tin dioxide (SnO
Thin Solid Films, 251, 19-19 (1994)
Xiao Han et al.
Small (Weinheim an der Bergstrasse, Germany), 15(39), e1901650-e1901650 (2019-08-03)
Long-term instability and possible lead contamination are the two main issues limiting the widespread application of organic-inorganic lead halide perovskites. Here a facile and efficient solution-phase method is demonstrated to synthesize lead-free Cs2 SnX6 (X = Br, I) with a
Keiko Yakabi et al.
ChemSusChem, 10(18), 3652-3659 (2017-08-15)
The Baeyer-Villiger oxidation is a key transformation for sustainable chemical synthesis, especially when H
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service