All Photos(1)
About This Item
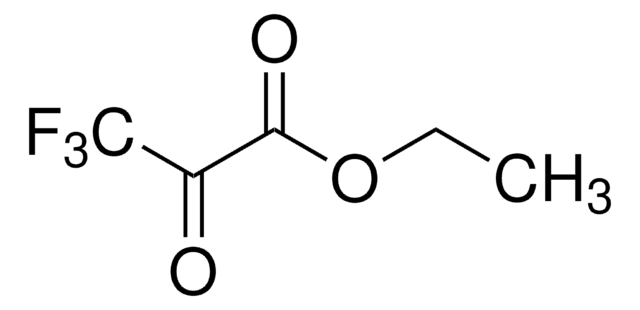
Linear Formula:
C2F5CO2C2H5
CAS Number:
Molecular Weight:
192.08
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.301 (lit.)
bp
75-76 °C (lit.)
density
1.299 g/mL at 25 °C (lit.)
functional group
ester
fluoro
SMILES string
CCOC(=O)C(F)(F)C(F)(F)F
InChI
1S/C5H5F5O2/c1-2-12-3(11)4(6,7)5(8,9)10/h2H2,1H3
InChI key
DBOFMRQAMAZKQY-UHFFFAOYSA-N
Application
Ethyl pentafluoropropionate has been used:
- as pentafluoroethyl source in the direct synthesis of pentafluoroethyl copper (CuC2F5)
- in the synthesis of C-6 substituted fluoroalkenyl 2,4-dimethoxypyrimidine derivative
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
35.6 °F - closed cup
Flash Point(C)
2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and structural characterization of the C-6 fluoroalkylated pyrimidine derivatives.
Kristafor S, et al.
Journal of Molecular Structure, 923(1), 19-23 (2009)
Hiroki Serizawa et al.
Organic letters, 16(13), 3456-3459 (2014-06-14)
The direct synthesis of pentafluoroethyl copper (CuC2F5) from a cuprate reagent and ethyl pentafluoropropionate as one of the most economical and useful pentafluoroethyl sources was accomplished. The advantages of this method are; all the reagents employed are low-cost and operationally
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service