287512
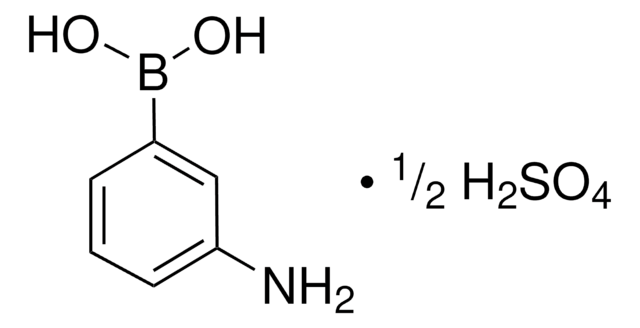
3-Aminophenylboronic acid monohydrate
98%
Synonym(s):
3-Aminobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H4B(OH)2 · H2O
CAS Number:
Molecular Weight:
154.96
Beilstein:
2936342
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
93-96 °C (lit.)
SMILES string
[H]O[H].Nc1cccc(c1)B(O)O
InChI
1S/C6H8BNO2.H2O/c8-6-3-1-2-5(4-6)7(9)10;/h1-4,9-10H,8H2;1H2
InChI key
XAEOVQODHLLNKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent used for
Reagent used for Preparation of
- Suzuki-Miyaura cross-coupling
Reagent used for Preparation of
- Gram-positive antivirulence drugs and inhibitors of Streptococcus agalactiae Stk1
- Regioisomer of Zaleplon (a sedative)
- Amphiphilic random glycopolymer, which self-assemble to form nanoparticles, with potential as a glucose-sensitive matrix
- Chemomechanical polymer that expands and contracts in blood plasma with high glucose selectivity
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic studies connected with the preparation of N-[3-(3-cyanopyrazolo[1,5-a]pyrimidin-5-yl)phenyl]-N-ethylacetamide, a zaleplon regioisomer
Radl, S.; et al.
Heterocycles, 80, 1359-1379 (2010)
Qianqian Guo et al.
Journal of biomaterials science. Polymer edition, 30(10), 815-831 (2019-05-03)
We reported on the fabrication of sugar-responsive nanogels covalently incorporated with 3-acrylamidophenylboronic acid (AAPBA) as glucose-recognizing moiety, 2-(acrylamido)glucopyranose (AGA) as biocompatible moiety, and boron dipyrromethene (BODIPYMA) as fluorescence donor molecule. The p(AAPBA-AGA-BODIPYMA) nanogels were synthesized via reversible addition-fragmentation chain transfer
Xingju Jin et al.
Biomacromolecules, 10(6), 1337-1345 (2009-04-29)
This study is devoted to developing amphiphilic, random glycopolymers based on phenylboronic acid, which self-assemble to form nanoparticles (NPs), as a glucose-sensitive agent. Maleimide-glucosamine was copolymerized with 3-acryl aminophenylboronic acid in methanol at 70 degrees C. Using the nanoprecipitation method
Jiangying Zhu et al.
Materials science & engineering. C, Materials for biological applications, 117, 111273-111273 (2020-09-14)
In this work, poly(ethylene glycol)-b-poly[3-acrylamidophenylboronic acid-co-styrene] (PEG-b-P(PBA-co-St) has been firstly synthesized for loading of insulin to form insulin-loaded micelles. Insulin-loaded micelles (ILM) and epidermal growth factor (EGF) are further embedded into the composite hydrogels that can be rapidly gelled by
A chemomechanical polymer that functions in blood plasma with high glucose selectivity.
George K Samoei et al.
Angewandte Chemie (International ed. in English), 45(32), 5319-5322 (2006-08-24)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service