All Photos(2)
About This Item
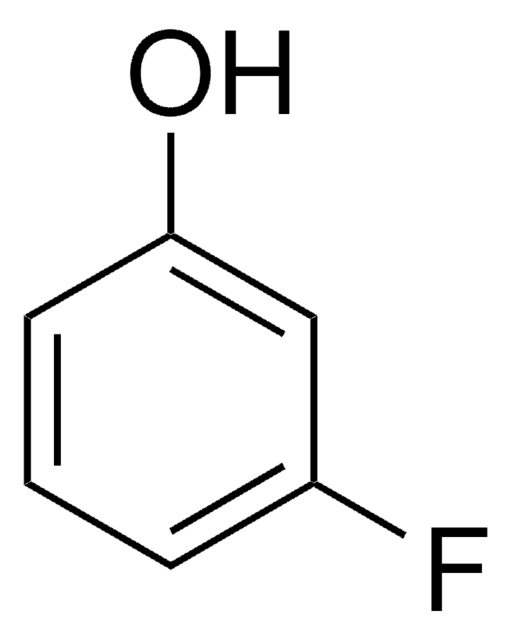
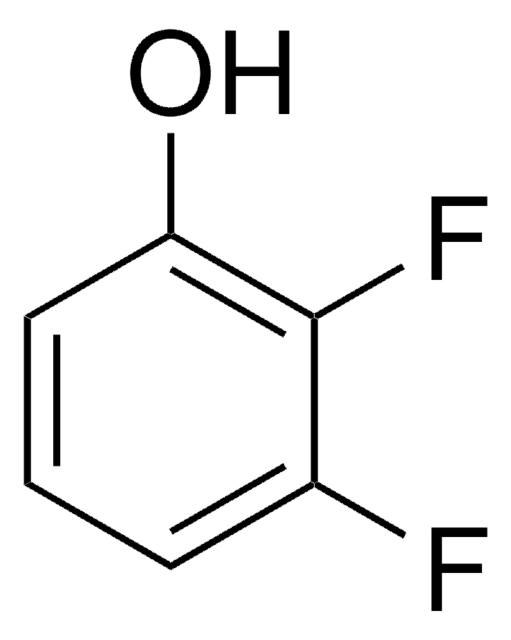
Linear Formula:
F2C6H3OH
CAS Number:
Molecular Weight:
130.09
Beilstein:
2043613
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
storage condition
protect from light
bp
59-61 °C/17 mmHg (lit.)
mp
38-41 °C (lit.)
solubility
ethanol: soluble 50 mg/mL, clear, colorless to light yellow
functional group
fluoro
storage temp.
2-8°C
SMILES string
Oc1c(F)cccc1F
InChI
1S/C6H4F2O/c7-4-2-1-3-5(8)6(4)9/h1-3,9H
InChI key
CKKOVFGIBXCEIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-difluorophenol undergoes oxidative polymerization in the presence of the Fe-N,N′-bis(salicylidene)ethylenediamine (salen) complex (catalyst) and hydrogen peroxide (oxidizing agent) to give poly(2,6-difluoro-1,4-phenylene oxide).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oxidative Polymerization of 2,6-Difluorophenol to Crystalline Poly(2,6-difluoro-1,4-phenylene oxide).
Ikeda R, et al.
Macromolecules, 33(18), 6648-6652 (2000)
J Qiu et al.
Journal of medicinal chemistry, 42(2), 329-332 (1999-02-02)
3-(Aminomethyl)-2,6-difluorophenol (6) and 4-(aminomethyl)-2, 6-difluorophenol (7) were synthesized in eight and four steps, respectively, starting from 2,6-difluorophenol, to test the potential of the 2,6-difluorophenol moiety to act as a lipophilic bioisostere of a carboxylic acid. Compounds 6 and 7 are
Jiasong Li et al.
Biochemistry, 58(17), 2218-2227 (2019-04-05)
Cysteine dioxygenase (CDO) is a nonheme iron enzyme that adds two oxygen atoms from dioxygen to the sulfur atom of l-cysteine. Adjacent to the iron site of mammalian CDO, there is a post-translationally generated Cys-Tyr cofactor, whose presence substantially enhances
Akira Ohashi et al.
Talanta, 146, 789-794 (2015-12-24)
The solubilities of several cobalt(III) chelates (CoL3) with in supercritical carbon dioxide (SC-CO2) were investigated in the presence of fluorine- and trifluoromethyl-substituted phenols (PhOH) using UV-vis spectrophotometry. Solubility enhancement of CoL3 complexes in SC-CO2 was accomplished by adding PhOH. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service