All Photos(1)
About This Item
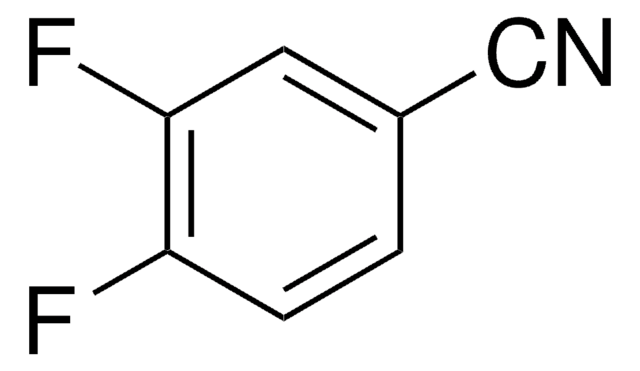
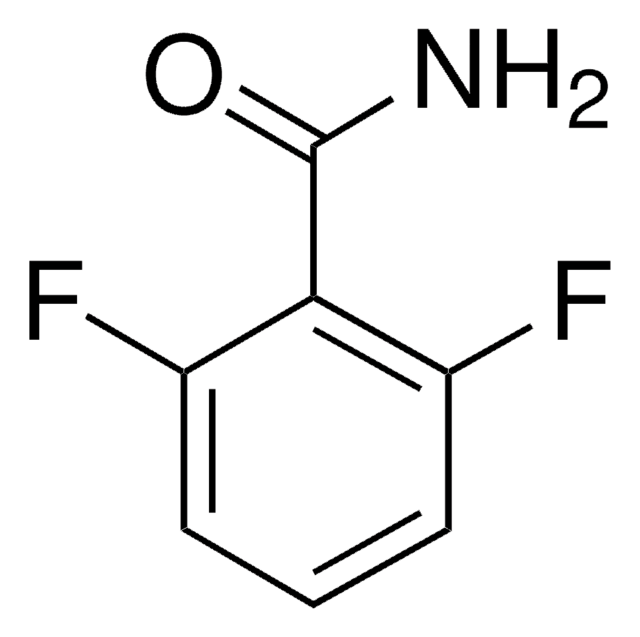
Linear Formula:
F2C6H3CN
CAS Number:
Molecular Weight:
139.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
47-49 °C (lit.)
functional group
fluoro
nitrile
SMILES string
Fc1ccc(C#N)c(F)c1
InChI
1S/C7H3F2N/c8-6-2-1-5(4-10)7(9)3-6/h1-3H
InChI key
LJFDXXUKKMEQKE-UHFFFAOYSA-N
General description
2,4-Difluorobenzonitrile undergoes polycondensation with Bisphenol A and matrix-assisted laser desorption/time-of-flight mass spectra revealed a quantitative formation of cyclic oligoethers and polyethers.
Application
2,4-Difluorobenzonitrile has been used in the synthesis of:
- 2-methylsulfonyl-4-fluorobenzylamine
- fluoro-3-amino-1,2-benzisoxazoles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Macrocycles. XXVIII. Cyclic poly (benzonitrile ether) s derived from bisphenol A.
Kricheldorf HR, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 41(23), 3838-3846 (2003)
Practical Synthesis of 4-Fluoro-2-(methylthio) benzylamine and the Corresponding Sulfone and Sulfonamide.
Perlow DS, et al.
Synthetic Communications, 37(11), 1887-1897 (2007)
S D Lepore et al.
The Journal of organic chemistry, 65(10), 2924-2932 (2000-05-18)
Further exploration of the scope of our solid-phase method for the synthesis of 3-aminobenzisoxazoles (using the Kaiser oxime resin 1) is described. The effects of base, leaving group, and solvent on the nucleophilic aromatic substitution based resin-loading reaction are discussed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service