263761
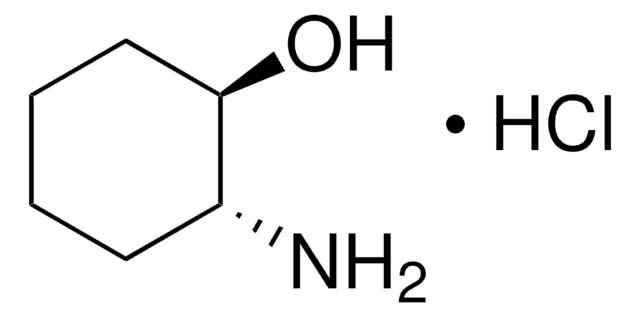
trans-4-Aminocyclohexanol hydrochloride
97%
Synonym(s):
trans-4-Hydroxycyclohexylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H10OH · HCl
CAS Number:
Molecular Weight:
151.63
Beilstein:
3909294
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
225-227 °C (lit.)
functional group
hydroxyl
SMILES string
Cl.N[C@H]1CC[C@H](O)CC1
InChI
1S/C6H13NO.ClH/c7-5-1-3-6(8)4-2-5;/h5-6,8H,1-4,7H2;1H/t5-,6-;
InChI key
RKTQEVMZBCBOSB-KYOXOEKESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
trans-4-Aminocyclohexanol hydrochloride has been used in the synthesis of:
- N-substituted 7-azabicyclo[2.2.1]heptanes via transannular nucleophilic displacement
- trans-4-methoxyoxalamido-1-cyclohexanol
- benzoxazine, required for the preparation of polybenzoxazine-silica hybrid nanocomposites
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D M Creasy et al.
Experimental and molecular pathology, 52(2), 155-169 (1990-04-01)
Male Wistar strain rats were fed a diet providing an intake of 0 or 400 mg cyclohexylamine (CHA)/kg body weight/day for 1, 3, 7, 9, or 13 weeks. At the end of the appropriate feeding period the rats were perfused-fixed
Polybenzoxazine-silica (PBZ-SiO2) hybrid nanocomposites through in situ sol-gel method.
Devaraju S, et al.
Journal of Sol-Gel Science and Technology, 60(1), 33-40 (2011)
Synthesis and microbial hydroxylation of some azabicycloalkanes.
Olivo HF, et al.
Tetrahedron Letters, 39(11), 1309-1312 (1998)
Dwayne A Dias et al.
Organic letters, 11(16), 3694-3697 (2009-07-28)
The intramolecular variant of the homo-[3 + 2]-dipolar cycloaddition of nitrones (generated in situ from an aldehyde and a hydroxylamine) with donor-acceptor cyclopropanes allows for the efficient synthesis of bridged tetrahydro-1,2-oxazines. This domino sequence produces adducts amenable to reductive N-O
N N Polushin
Nucleic acids research, 28(16), 3125-3133 (2000-08-10)
Synthesis of new terminus modifiers, bearing, along with a phosphoramidite moiety, one, two or four methoxyoxalamido (MOX) precursor groups, is described. These modifiers are introduced onto the 5'-end of a synthetic oligodeoxyribonucleotide as the last step of an automated synthesis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service