All Photos(1)
About This Item
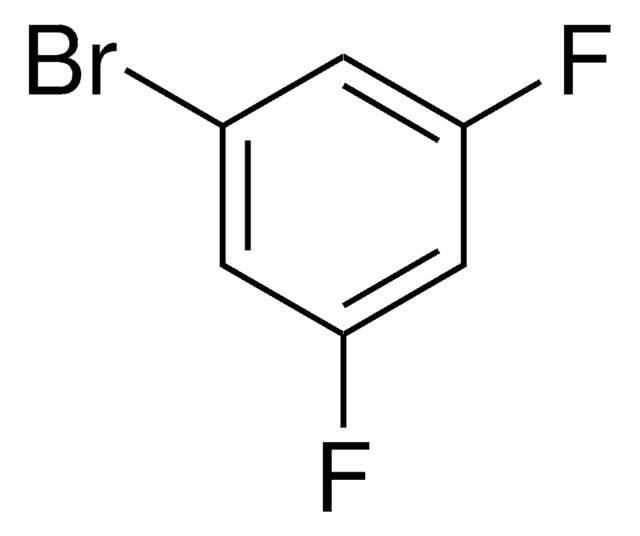
Linear Formula:
BrC6H3F2
CAS Number:
Molecular Weight:
192.99
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.51 (lit.)
functional group
bromo
fluoro
SMILES string
Fc1cccc(F)c1Br
InChI
1S/C6H3BrF2/c7-6-4(8)2-1-3-5(6)9/h1-3H
InChI key
HRZTZLCMURHWFY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Three-component coupling reaction of 1-bromo-2,6-difluorobenzene with benzyne and isocyanides has been reported.
Application
1-Bromo-2,6-difluorobenzene has been used in the synthesis of tris(fluorophenyl)boranes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
127.4 °F - closed cup
Flash Point(C)
53 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Juan A Nicasio et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(33), 11016-11020 (2013-07-03)
An analysis of the metal-free reduction of electron deficient olefins by frustrated Lewis pairs indicates that the rate-determining step might be either the heterolytic cleavage of H2 to form an -onium borohydride salt, or the subsequent transfer of the hydride
Three-component coupling of arynes and organic bromides.
Hiroto Yoshida et al.
Angewandte Chemie (International ed. in English), 50(41), 9676-9679 (2011-09-03)
Ji Gwang Yu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(63), 16044-16050 (2017-08-24)
Four dibenzofuran-type host materials substituted with a carbazolylcarbazole moiety were synthesized to investigate the effect of substitution position on the material parameters and device performances of host materials. The carbazolylcarbazole moiety was substituted at the 1-, 2-, 3-, and 4-positions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service