254738
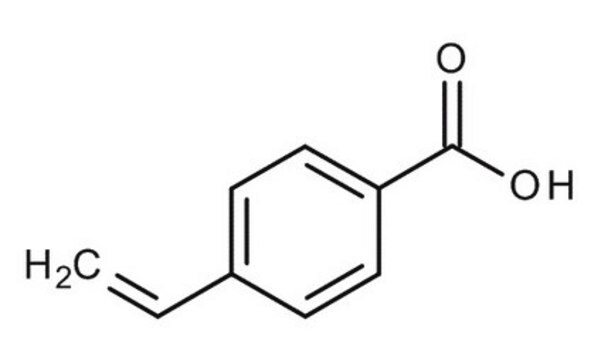
4-Vinylbenzoic acid
97%
Synonym(s):
Styrene-4-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=CHC6H4CO2H
CAS Number:
Molecular Weight:
148.16
Beilstein:
2041130
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
mp
142-144 °C (lit.)
SMILES string
OC(=O)c1ccc(C=C)cc1
InChI
1S/C9H8O2/c1-2-7-3-5-8(6-4-7)9(10)11/h2-6H,1H2,(H,10,11)
InChI key
IRQWEODKXLDORP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Vinylbenzoic acid (4-VBA), also known as styrene-4-carboxylic acid, is an aromatic compound characterized by a vinyl group attached to the para position of benzoic acid. Its vinyl group allows for free radical polymerization, facilitating the formation of various copolymers and hydrogels, making it a versatile monomer in materials science. 4-VBA exhibits good thermal stability, which is essential for applications requiring high-temperature processing and stability. Additionally, 4-VBA can also be modified to enhance its biocompatibility, making it suitable for drug delivery systems and tissue engineering.
Application
4-Vinylbenzoic acid can be used as:
- A capping agent in the preparation of polyrotaxane hydrogels. The incorporation of 4-VBA modifies and enhances the structural integrity and functionality of the resulting hydrogels, rendering them suitable for biological applications.
- A monomer in the synthesis of molecularly imprinted polymers (MIPs). The incorporation of 4-VBA contributes to improved selectivity in MIPs. The study highlights that MIPs synthesized with 4-VBA exhibit significant binding affinity towards specific biomolecules, making them effective for applications such as drug delivery and biosensing.
- A functional monomer in the synthesis of benzoic acid-modified monolithic columns. These columns are suitable for a wide range of applications, including drug analysis and environmental monitoring, where the separation of small, polar molecules is often challenging.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chen Liang et al.
Journal of agricultural and food chemistry, 66(38), 10086-10096 (2018-09-18)
Polymeric sorbents are frequently used in wine, either as solid phase extraction materials for isolation of analytes or as sorptive materials for removal of undesirable compounds (amelioration). Six new polymeric sorbents were produced thermally or in a microwave from various
Ghadeer F Abu-Alsoud et al.
Journal of chromatography. A, 1629, 461463-461463 (2020-08-26)
Cross-reactivity is an important feature of molecularly imprinted polymers (MIPs), and is central to successful use of a pseudo-template in molecular imprinting. The adsorption and cross-reactivity of a molecularly imprinted polymer (MIP) designed for recognition of phenols from water was
Sung-Hwan Lim et al.
Nature communications, 8(1), 360-360 (2017-08-27)
Synthesis of binary nanoparticle superlattices has attracted attention for a broad spectrum of potential applications. However, this has remained challenging for one-dimensional nanoparticle systems. In this study, we investigate the packing behavior of one-dimensional nanoparticles of different diameters into a
Marcin Woźnica et al.
Journal of separation science, 42(7), 1412-1422 (2019-01-27)
The objective of this article was to design the selective molecularly imprinted sorbent dedicated to the solid-phase extraction of S-pramipexole from the complex matrix such as human urine. For that purpose, S-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole was used as the template acting as the
Monika Sobiech et al.
Journal of chromatography. A, 1613, 460677-460677 (2019-11-16)
The objective of this paper was to extend comprehensive theoretical and experimental investigations at the molecular level to identify factors responsible for the high selectivity of imprinted sorbents. This knowledge was utilized in a new analytical strategy devoted to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service