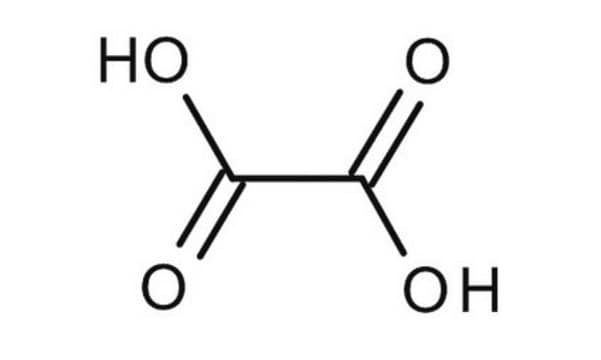
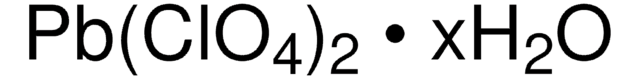241172
Oxalic acid
ReagentPlus®, ≥99%
Synonym(s):
Aktisal, Aquisal
About This Item
Recommended Products
vapor density
4.4 (vs air)
vapor pressure
<0.01 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
≥99%
form
powder or crystals
mp
189.5 °C (dec.) (lit.)
solubility
water: soluble ~108 g/L at 25 °C
density
1.65 g/cm3 at 18.5 °C
functional group
carboxylic acid
SMILES string
OC(=O)C(O)=O
InChI
1S/C2H2O4/c3-1(4)2(5)6/h(H,3,4)(H,5,6)
InChI key
MUBZPKHOEPUJKR-UHFFFAOYSA-N
Gene Information
human ... SRC(6714)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- 2-aryl-1-arylmethyl-1H-benzimidazoles from o-phenylenediamine and aldehydes
- 2-aryl-4,5-diphenyl-1H-imidazoles from benzyl/benzoin, ammonium acetate and aromatic aldehydes
- bis-compounds of indole and 2-naphthol in an aqueous medium
- in the synthesis of hemicellulose hydrolysates of yellow poplars
- in the synthesis of three-dimensionally ordered macroporous metal oxides or carbonates via templating with polystyrene spheres
- as supporting electrolyte in the electrochemical synthesis of polyaniline-polypyrrole composite coatings
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service