All Photos(1)
About This Item
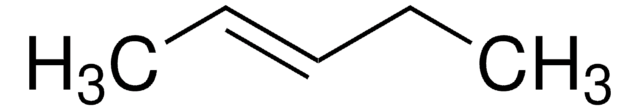
Linear Formula:
CH3(CH2)3CH=CH2
CAS Number:
Molecular Weight:
84.16
Beilstein:
1209240
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3 (vs air)
vapor pressure
155 mmHg ( 21.1 °C)
Assay
≥99%
form
liquid
autoignition temp.
487 °F
refractive index
n20/D 1.388 (lit.)
bp
60-66 °C (lit.)
density
0.678 g/mL at 25 °C (lit.)
functional group
allyl
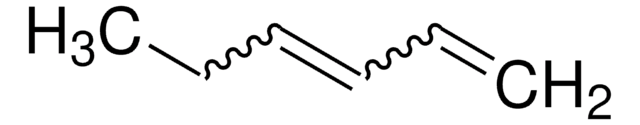
SMILES string
CCCCC=C
InChI
1S/C6H12/c1-3-5-6-4-2/h3H,1,4-6H2,2H3
InChI key
LIKMAJRDDDTEIG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The kinetics of polymerization of 1-hexene was studied using a family of five zirconium amine bis-phenolate catalysts. Polymerization reaction of 1-hexene catalyzed by a series of hafnium complexes was studied.
Application
- 1-Hexene in High Molecular Weight Polymer Synthesis: The synthesis of high molecular weight copolymers from 1-hexene and methyl acrylate using Lewis acid catalysts showcased advanced applications in materials science, specifically in developing durable and versatile polymer materials (Wan et al., 2024).
- 1-Hexene′s Role in Proton-Exchange Membrane Enhancement: Utilizing 1-hexene in the structural characterization and enhancement of physicochemical properties of functionally porous proton-exchange membranes highlights its critical role in improving energy efficiency and performance in fuel cell technologies (Ponomar et al., 2024).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
-13.0 °F - closed cup
Flash Point(C)
-25.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D Keith Steelman et al.
Journal of the American Chemical Society, 135(16), 6280-6288 (2013-03-23)
The kinetics of 1-hexene polymerization using a family of five zirconium amine bis-phenolate catalysts, Zr[tBu-ON(X)O]Bn2 (where X = THF (1), pyridine (2), NMe2 (3), furan (4), and SMe (5)), has been investigated to uncover the mechanistic effect of varying the
Takahiro Yasumoto et al.
Dalton transactions (Cambridge, England : 2003), 42(25), 9120-9128 (2012-12-14)
Non-bridged half-metallocene dimethyl complexes of group 4 metals 2a-4a with an N-4-methoxyphenyl(iminomethyl)pyrrolyl ligand 1a were synthesized and characterized by NMR spectroscopy and X-ray analysis. Upon activation with [Ph3C][B(C6F5)4], these complexes became active catalysts for the polymerization of 1-hexene. A series
Lingyan Gong et al.
Journal of colloid and interface science, 569, 219-228 (2020-03-01)
The current mechanism of surfactant enhanced oil recovery (EOR) mainly relies on forming middle-phase microemulsions to get ultra-low oil-water interfacial tension. However, residual oil can also be recovered using low concentration surfactant solutions without microemulsion formation, and the interaction between
Chia-Hsiu Chen et al.
Journal of the American Chemical Society, 137(21), 6965-6971 (2015-05-12)
The stereochemistry, kinetics, and mechanism of olefin polymerization catalyzed by a set of zirconium-based metallocenes was studied by NMR using dissolution dynamic nuclear polarization (DNP). Hyperpolarized 1-hexene was polymerized in situ with a C2 symmetric catalyst, [(EBI)ZrMe][B(C6F5)4] (EBI = rac-(C2H4(1-indenyl)2))
Yuichi Ito et al.
Implant dentistry, 24(4), 477-479 (2015-06-04)
The evaluation of bone quality at the site of the alveolar bone for a dental implant is very important. This study presents an easy technique for direct evaluation of alveolar bone quality using nondecalcified cryofilm frozen sections on human alveolar
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service