233633
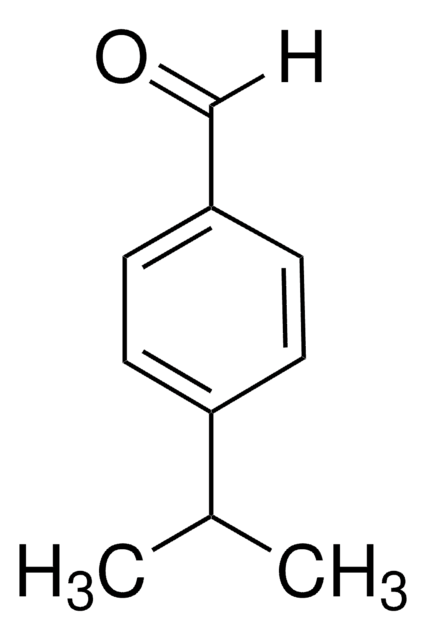
4-Ethylbenzaldehyde
98%
Synonym(s):
p-Ethylbenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C2H5C6H4CHO
CAS Number:
Molecular Weight:
134.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.539 (lit.)
bp
221 °C (lit.)
density
0.979 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(CC)cc1
InChI
1S/C9H10O/c1-2-8-3-5-9(7-10)6-4-8/h3-7H,2H2,1H3
InChI key
QNGNSVIICDLXHT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Ethylbenzaldehyde is a by-product of disinfection. Kinetic constants (KI) of 4-ethylbenzaldehyde for inhibition of the diphenolase activity of mushroom tyrosinase has been investigated.
Application
4-Ethylbenzaldehyde has been used in the synthesis of 4,4′-diaminotriphenylmethanes under microwave irradiation, which is useful for parallel library syntheses.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
197.6 °F - closed cup
Flash Point(C)
92 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-assisted synthesis of 4, 4'-diaminotriphenylmethanes.
Guzman-Lucero D, et al.
Tetrahedron Letters, 46(7), 1119-1122 (2005)
Gergely Rácz et al.
Pathology oncology research : POR, 18(3), 579-584 (2011-12-14)
Disinfection of raw water is essential to the production of drinking water. However, by-products of disinfection may exert toxic effects. The potential toxic effects of two of these compounds, 4-ethylbenzaldehyde (EBA) and 2,4-difluoroaniline (DFA) were investigated using the zebrafish (Danio
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Yunzi Feng et al.
Food chemistry, 265, 274-280 (2018-06-10)
Two types of chicken broth, broiler broth (BB) and native chicken broth (NCB), were used to analyse their differences in aroma by gas chromatography-olfactometry/mass spectrometry (GC-O/MS). NCB contained more complex volatiles and exhibited a richer aromatic profile compared with BB.
Eduardo Coelho et al.
Food research international (Ottawa, Ont.), 116, 249-257 (2019-02-06)
Cooperage wood is a porous material and beverages exchange compounds with it by penetrating into its pores. This work demonstrates the enrichment of wood with wine during ageing. Three oak varieties were cut into different sized chips and immersed in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service