All Photos(1)
About This Item
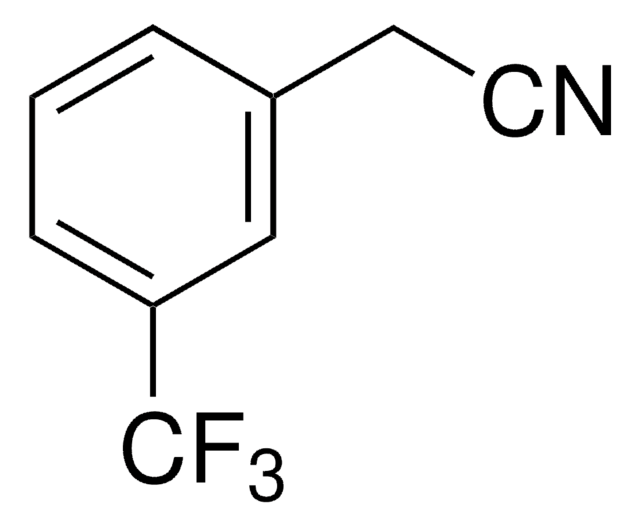
Linear Formula:
CF3C6H4CH2CN
CAS Number:
Molecular Weight:
185.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
131-132 °C/20 mmHg (lit.)
mp
47-49 °C (lit.)
functional group
fluoro
nitrile
SMILES string
FC(F)(F)c1ccc(CC#N)cc1
InChI
1S/C9H6F3N/c10-9(11,12)8-3-1-7(2-4-8)5-6-13/h1-4H,5H2
InChI key
QNKOCFJZJWOXDE-UHFFFAOYSA-N
Related Categories
Application
4-(Trifluoromethyl)phenylacetonitrile was used in the preparation of:
- 1,4-bis[2-cyano-2-(4-(trifluoromethyl)phenyl)vinyl]benzene
- cyano-substituted distyrylbenzene derivative, novel n-type organic semiconductor
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Steady Operation of n-Type Organic Thin-Film Transistors with Cyano-Substituted Distyrylbenzene Derivative.
Nagamatsu S, et al.
Applied Physics Express, 2(10), 101502-101502 (2009)
Synthesis and FET characteristics of phenylene-vinylene and anthracene-vinylene compounds containing cyano groups.
Shoji K, et al.
Journal of Materials Chemistry, 20(31), 6472-6478 (2010)
Immacolata Serra et al.
Marine biotechnology (New York, N.Y.), 21(2), 229-239 (2019-01-27)
A screening among marine yeasts was carried out for nitrile hydrolyzing activity. Meyerozyma guilliermondii LM2 (UBOCC-A-214008) was able to efficiently grow on benzonitrile and cyclohexanecarbonitrile (CECN) as sole nitrogen sources. A two-step one-pot method for obtaining cells of M. guilliermondii
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service