223662
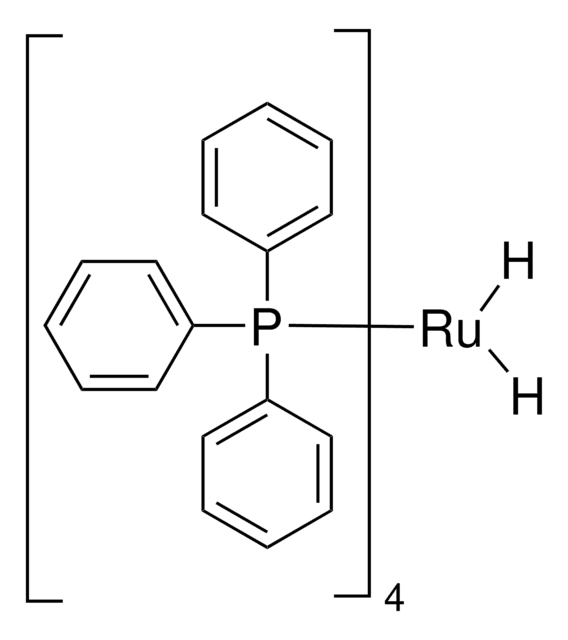
Tris(triphenylphosphine)ruthenium(II) dichloride
97%
Synonym(s):
Tris(triphenylphosphine)dichlororuthenium, Dichlorotris(triphenylphosphine)ruthenium(II), Ruthenium(II)-tris(triphenylphosphine) dichloride
About This Item
Recommended Products
Quality Level
Assay
97%
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: C-H Activation
SMILES string
Cl[Ru]Cl.c1ccc(cc1)P(c2ccccc2)c3ccccc3.c4ccc(cc4)P(c5ccccc5)c6ccccc6.c7ccc(cc7)P(c8ccccc8)c9ccccc9
InChI
1S/3C18H15P.2ClH.Ru/c3*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h3*1-15H;2*1H;/q;;;;;+2/p-2
InChI key
WIWBLJMBLGWSIN-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
- Functionalized alcohols by C-C cross-coupling reaction between different alcohols via sp3 C-H bond activation of primary alcohols in the presence of Lewis acid.
- Furan derivatives from allenyl sulfides via 1,4 migration of the sulfanyl group.
- 1,3-diphenylpropan-1-one by alkylation of acetophenone with benzyl alcohol via C-C bond formation.
- Vinyl chloride monomer by hydrochlorination reaction of acetylene.
RuCl2(PPh3)3 can also be used as a catalyst in the cyclization, isomerization, reduction, oxidation, and cross-coupling reactions of a variety of organic products. Hydrogenation of nitro groups, imines, and ketones, as well as selective oxidation of alcohols are also possible in the presence of this catalyst.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service