All Photos(1)
About This Item
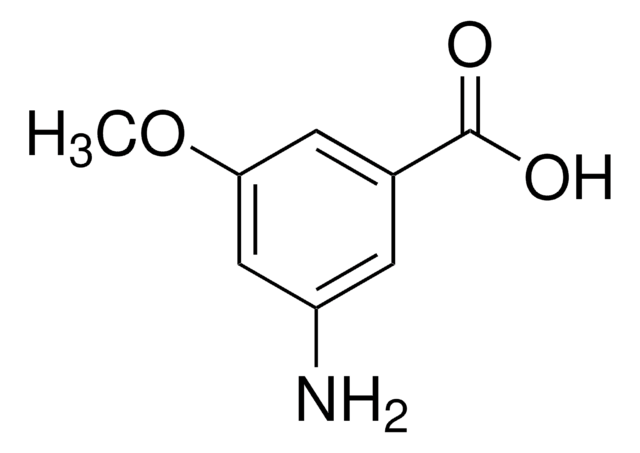
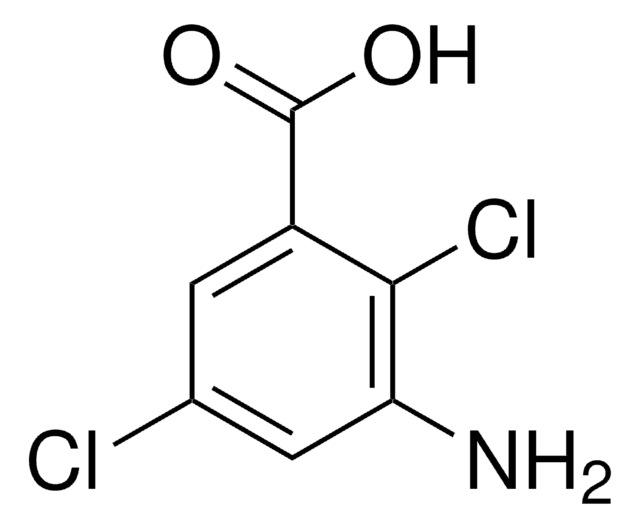
Linear Formula:
H2NC6H3(Cl)CO2H
CAS Number:
Molecular Weight:
171.58
Beilstein:
2803668
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: solution phase peptide synthesis
mp
211 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
Nc1ccc(C(O)=O)c(Cl)c1
InChI
1S/C7H6ClNO2/c8-6-3-4(9)1-2-5(6)7(10)11/h1-3H,9H2,(H,10,11)
InChI key
MBDUKNCPOPMRJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R R Weiss et al.
Anesthesia and analgesia, 62(2), 168-173 (1983-02-01)
2-Chloroprocaine (CP) has recently been recommended as a less toxic alternative to amide-type local anesthetics due to its rapid metabolism. A double-blind, randomized study comparing CP to lidocaine when used for paracervical block was carried out. Twenty-nine patients received CP
B R Kuhnert et al.
Anesthesia and analgesia, 62(12), 1089-1094 (1983-12-01)
Little is known about the pharmacology of the metabolites of 2-chloroprocaine in obstetrical patients. The primary objective of this study was to describe the elimination of 2-chloroaminobenzoic acid (CABA) in maternal and neonatal urine after epidural anesthesia. A secondary objective
E H Philipson et al.
American journal of obstetrics and gynecology, 151(3), 322-324 (1985-02-01)
Amide-linked local anesthetic agents, such as lidocaine and bupivacaine, can become "trapped" in their ionized forms on the fetal side of the placenta, and therefore their net transfer across the placenta is increased. An ester-linked local anesthetic agent, 2-chloroprocaine, is
G Menon et al.
Journal of pharmaceutical sciences, 73(2), 251-253 (1984-02-01)
A high-performance liquid chromatographic method has been developed for the simultaneous determination of chloroprocaine hydrochloride and its hydrolytic degradation product, 4-amino-2-chlorobenzoic acid. Separation is achieved using a mu-Bondapak C18 column and the eluant, water-acetonitrile-methanol-glacial acetic acid (74:20:5:1) containing 0.05-0.08% (w/v)
F Brun et al.
Journal of pharmaceutical and biomedical analysis, 14(8-10), 1251-1259 (1996-06-01)
The separation of HPLC of basic drugs on silica-based reversed phases remains a major problem because of the interaction between the residual silanol groups of the silica and the amino function of the drug. This paper describes the validation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service