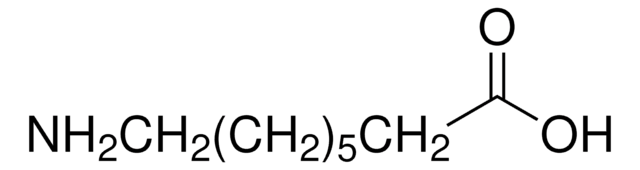217700
DL-2-Aminocaprylic acid
99%
Synonym(s):
DL-2-Aminooctanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
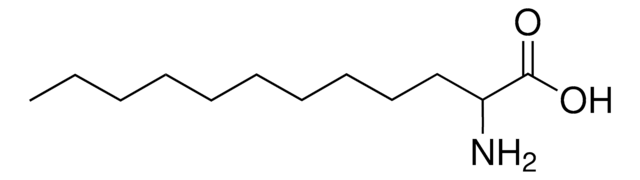
CH3(CH2)5CH(NH2)CO2H
CAS Number:
Molecular Weight:
159.23
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
260 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
CCCCCCC(N)C(O)=O
InChI
1S/C8H17NO2/c1-2-3-4-5-6-7(9)8(10)11/h7H,2-6,9H2,1H3,(H,10,11)
InChI key
AKVBCGQVQXPRLD-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Paper chromatography of 56 amino compounds using phenol and butanol-acetic acid as solvents with illustrative chromatograms of normal and abnormal urines.
T E PARRY
Clinica chimica acta; international journal of clinical chemistry, 2(2), 115-125 (1957-04-01)
Milica Markovic et al.
Pharmaceutics, 11(4) (2019-04-19)
In ulcerative colitis (UC), the inflammation is localized in the colon, and one of the successful strategies for colon-targeting drug delivery is the prodrug approach. In this work, we present a novel phospholipid (PL)-based prodrug approach, as a tool for
Arik Dahan et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 108, 78-85 (2017-06-20)
The enzyme phospholipase A
Seon-Hee Kim et al.
PLoS neglected tropical diseases, 6(10), e1868-e1868 (2012-11-15)
Fatty acid (FA) binding proteins (FABPs) of helminths are implicated in acquisition and utilization of host-derived hydrophobic substances, as well as in signaling and cellular interactions. We previously demonstrated that secretory hydrophobic ligand binding proteins (HLBPs) of Taenia solium metacestode
Sarah A Almahboub et al.
Applied microbiology and biotechnology, 102(2), 789-799 (2017-11-28)
Terminal modification of peptides is frequently used to improve their hydrophobicity. While N-terminal modification with fatty acids (lipidation) has been reported previously, C-terminal lipidation is limited as it requires the use of linkers. Here we report the use of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service