216143
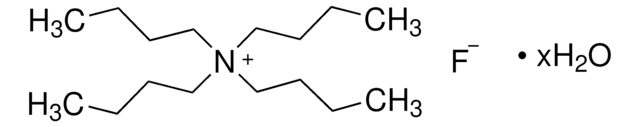
Tetrabutylammonium fluoride solution
1.0 M in THF
Synonym(s):
TBAF solution
About This Item
Recommended Products
form
liquid
Quality Level
concentration
1.0 M in THF
impurities
~5 wt. % water
density
0.903 g/mL at 25 °C
functional group
amine
storage temp.
2-8°C
SMILES string
[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
InChI key
FPGGTKZVZWFYPV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Triple monoamine reuptake inhibitors as a new generation of antidepressants
- Alcohols via hydrolysis of alkyl silyl ethers neutral pH in mixed organic-aqueous buffered solutions
- Oligoribonucleotides with phosphonate-modified linkages
- Aryl alkyl alcohols via Nozaki-Hiyama allylation catalyzed by chiral bipyridyldiol ligands and chromium trichloride
- Conjugated dienoic acid esters using Suzuki coupling reactions
- Macrocyclic o-aminobenzamide Hsp90 inhibitorwith antitumor activity
- Phospshoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors
- Anti-diabetic polyacetylenic glucosides
- For the deprotection of silyl and N-sulfonyl groups.
- In the fluorination reactions.
- To synthesize 2-substituted indoles by cyclization reaction of various 2-ethynylanilines with terminal alkynes using Pd catalyst. It can also be used as an activator in the synthesis of arylated or alkenylated alkynes by the coupling reaction of aryl and alkenyl halides with terminal alkynes in the presence of Pd catalyst.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service