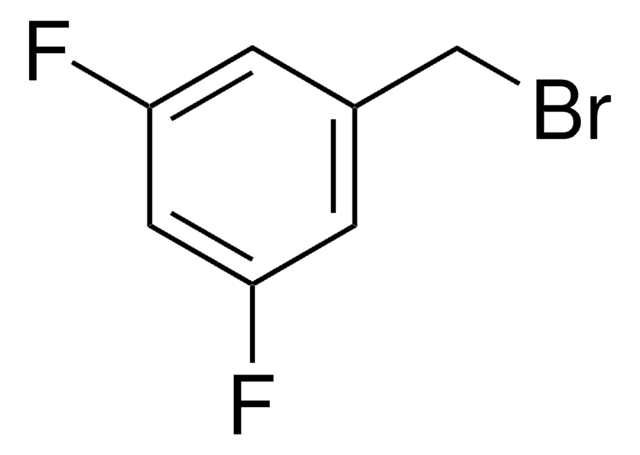
209538
4-Fluorobenzyl bromide
97%
Synonym(s):
α-Bromo-4-fluorotoluene, 1-Bromomethyl-4-fluorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4CH2Br
CAS Number:
Molecular Weight:
189.02
Beilstein:
636507
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.547 (lit.)
bp
85 °C/15 mmHg (lit.)
density
1.517 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1ccc(CBr)cc1
InChI
1S/C7H6BrF/c8-5-6-1-3-7(9)4-2-6/h1-4H,5H2
InChI key
NVNPLEPBDPJYRZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Fluorobenzyl bromide was used in the preparation of:
- 3-acetyl-1-(4-fluorobenzyl)-4-hydroxy-1H-indole
- 1-(4-fluorobenzyl)-5-(pyrrolidine-1-sulfonyl)isatin
- (S)-1-(4-fluorobenzyl)-5-(2-(4-fluorophenoxymethyl)pyrrolidine-1-sulfonyl)isatin
- 8-bromo 9-(4-fluorobenzyl) adenine
- 8-bromo 3-(4-fluorobenzyl) adenine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
>235.4 °F - closed cup
Flash Point(C)
> 113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Graham Smith et al.
Journal of medicinal chemistry, 51(24), 8057-8067 (2008-12-04)
Imaging of programmed cell death (apoptosis) is important in the assessment of therapeutic response in oncology and for diagnosis in cardiac and neurodegenerative disorders. The executioner caspases 3 and 7 ultimately effect cellular death, thus providing selective molecular targets for
Laura De Luca et al.
ChemMedChem, 4(8), 1311-1316 (2009-07-01)
The cellular protein lens epithelium-derived growth factor, or transcriptional coactivator p75 (LEDGF/p75), plays a crucial role in HIV integration. The protein-protein interactions (PPIs) between HIV-1 integrase (IN) and its cellular cofactor LEDGF/p75 may therefore serve as targets for the development
Xingnan Li et al.
Bioorganic & medicinal chemistry, 14(16), 5742-5755 (2006-06-07)
Because of its essential role in HIV replication and lack of human counterpart, HIV integrase is an attractive target for the development of novel anti-AIDS agents. Among the recently developed integrase inhibitors, only the alpha,gamma-diketo acid (DKA) compounds were biologically
Kathleen E Prosser et al.
Journal of inorganic biochemistry, 167, 89-99 (2016-12-05)
The Cu(II) complex CuCl
Ihor Kulai et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(63), 16066-16077 (2017-08-31)
Eight alkyl triarylstannanecarbodithioates were synthesized starting from the corresponding triarylstannyl chlorides. They were fully characterized by IR and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service