All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H6O
CAS Number:
Molecular Weight:
70.09
Beilstein:
103168
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
14.46 psi ( 55 °C)
3.67 psi ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
54-55 °C (lit.)
density
0.927 g/mL at 25 °C (lit.)
functional group
ether
storage temp.
2-8°C
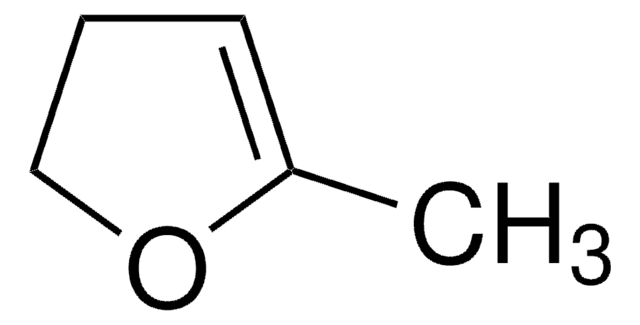
SMILES string
C1CC=CO1
InChI
1S/C4H6O/c1-2-4-5-3-1/h1,3H,2,4H2
InChI key
JKTCBAGSMQIFNL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The enantioselective Heck arylation of 2,3-dihydrofuran with aryl iodides was studied.
Application
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-11.2 °F - closed cup
Flash Point(C)
-24 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The Journal of Organic Chemistry, 59, 4535-4535 (1994)
Tetrahedron, 50, 4557-4557 (1994)
Synlett, 431-431 (1994)
Claudia G Cobo-Angel et al.
Scientific reports, 9(1), 14025-14025 (2019-10-03)
Group B Streptococcus (GBS), is a leading cause of neonatal death and an emerging pathogen in adults. Additionally, GBS is a bovine pathogen causing intramammary infections. The likelihood of GBS interspecies transmission is largely unknown. We explored the potential transmission
Qi-Fang Wang et al.
The Journal of organic chemistry, 74(19), 7403-7406 (2009-09-11)
An efficient synthetic procedure for the preparation of fused 2,3-dihydrofuran derivatives was developed with the assistance of pyridinium ylide. A sequential one-pot, two-step tandem reaction starting from pyridine, aromatic aldehyde, dimedone, or 4-hydroxycoumarin and alpha-phenacyl bromide or p-nitrobenzyl bromide with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service