196274
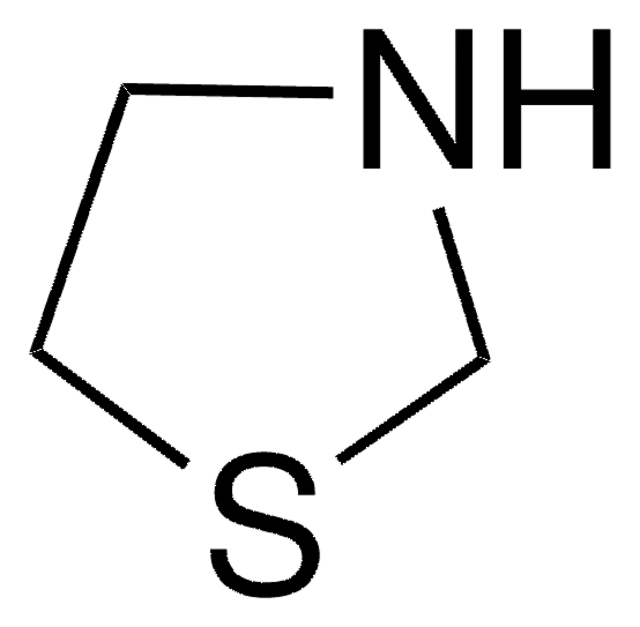
Thiomorpholine
98%
Synonym(s):
Tetrahydro-2H-1,4-thiazine, Thiamorpholine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H9NS
CAS Number:
Molecular Weight:
103.19
Beilstein:
102550
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.538 (lit.)
bp
169 °C (lit.)
solubility
organic solvents: miscible(lit.)
water: miscible(lit.)
density
1.026 g/mL at 25 °C (lit.)
functional group
thioether
SMILES string
C1CSCCN1
InChI
1S/C4H9NS/c1-3-6-4-2-5-1/h5H,1-4H2
InChI key
BRNULMACUQOKMR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Thiomorpholine forms complexes with Cu(II), Pt(II) and Ni(II) salts and their catalytic activity has been investigated.
Application
Thiomorpholine has been used in the preparation of:
- N-Boc-α-alkyl-β-(sec-amino)alanines
- thiomorpholine-N-borane
Signal Word
Danger
Hazard Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mesut Kacan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 118, 572-577 (2013-10-05)
Several Cu(II), Pt(II) and Ni(II) complexes of N-substituted, piperazine (NN donor), morpholine (NO donor) and thiomorpholine (NS donor) derivatives were synthesized and their thermal behavior and catalytic activity in epoxidation reaction of cis-diphenylethylene were studied using oxygen sources NaOCl. The
Synthesis and comparative reactivity of thiomorpholine-borane: aqueous hydrolysis and oxidation by hypochlorite.
Amezcua CA, et al.
Inorgorganica Chimica Acta, 290(1), 80-85 (1999)
J I Levin et al.
Bioorganic & medicinal chemistry letters, 16(6), 1605-1609 (2006-01-24)
A series of thiomorpholine sulfonamide hydroxamate TACE inhibitors, all bearing propargylic ether P1' groups, was explored. In particular, compound 5h has excellent in vitro potency against isolated TACE enzyme and in cells, oral activity in a model of TNF-alpha production
Upinder Singh et al.
Bioorganic & medicinal chemistry letters, 13(23), 4209-4212 (2003-11-19)
Combinatorial libraries of N-acylated 5-(S)-aminomethyloxazolidinone derivatives of S-oxide and S,S-dioxide tetrahydro-4(2H)-thiopyranyl and thiomorpholine phenyloxazolidinone series have been synthesized on a solid phase and evaluated for antimicrobial activity. Several novel potent leads have been identified, including orally active oxazolidinones with enhanced
Saurav Bera et al.
ACS combinatorial science, 14(1), 1-4 (2011-12-01)
Diastereoselective trans-2,5-disubstituted amino acids derived diverse morpholines, piperazines and thiomorpholines were prepared in 30 min-1 h with high yields through iodine-mediated 6-exotrig type cyclization from a single common synthetic intermediate. The displacement of iodine with hydride ion gave a methyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service