195359
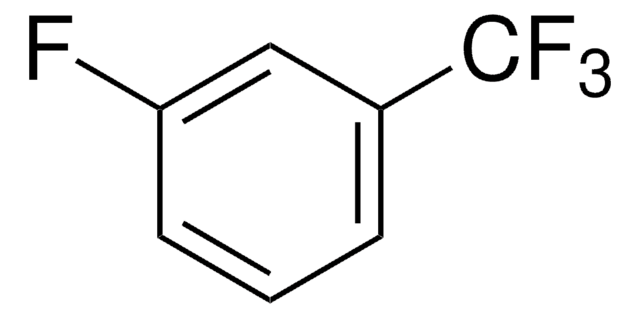
4-Fluorobenzotrifluoride
98%
Synonym(s):
α,α,α,4-Tetrafluorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4CF3
CAS Number:
Molecular Weight:
164.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.401 (lit.)
bp
102-105 °C (lit.)
mp
−42-−41.7 °C (lit.)
density
1.293 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccc(cc1)C(F)(F)F
InChI
1S/C7H4F4/c8-6-3-1-5(2-4-6)7(9,10)11/h1-4H
InChI key
UNNNAIWPDLRVRN-UHFFFAOYSA-N
Application
4-Fluorobenzotrifluoride was used in the synthesis of:
- 2,2′-bis(trifluoromethylphenoxy)biphenyl via nucleophilic aromatic substitution reaction with 2,2′-biphenol
- (S)-fluoxetine, potent inhibitor of neuronal serotonin-uptake
- 1-aryloxy-2-substituted aminomethyltetrahydronaphthalene derivatives
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D W Robertson et al.
Journal of medicinal chemistry, 31(7), 1412-1417 (1988-07-01)
Fluoxetine is a potent and selective inhibitor of the neuronal serotonin-uptake carrier and is a clinically effective antidepressant. Although fluoxetine is used therapeutically as the racemate, there appears to be a small but demonstrable stereospecificity associated with its interactions with
Kalpana Bhandari et al.
Bioorganic & medicinal chemistry, 14(8), 2535-2544 (2005-12-13)
Several 1-aryloxy-2-substituted aminomethyltetrahydronaphthalenes (7-21) as conformationally rigid analogues of fluoxetine were synthesized and evaluated for their anorexigenic and antidepressant activities. For SAR studies the related acyclic analogues (22-27) were also prepared. Out of the 21 synthesized compounds, 10 compounds (9
Akiko Okamoto et al.
Journal of oleo science, 56(9), 479-491 (2007-09-28)
Novel bifunctional acyl-acceptant biphenyls bearing trifluoromethylated aroyloxy groups were successfully synthesized in high yields via TfOH-mediated electrophilic aromatic aroylation of fluorobenzene with CF(3)-bearing aroyl chlorides followed by nucleophilic aromatic substitution with 2,2'-biphenol. Similarly, 2,2'-bis(trifluoromethylphenoxy)biphenyl was synthesized via nucleophilic aromatic substitution
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service