All Photos(3)
About This Item
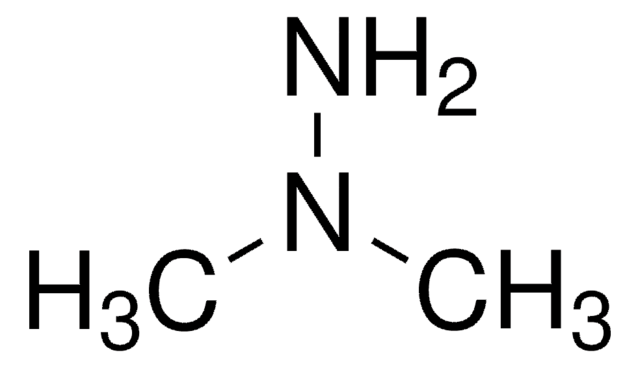
Linear Formula:
(CH3)3CNHNH2 · HCl
CAS Number:
Molecular Weight:
124.61
Beilstein:
3651224
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
191-194 °C (lit.)
functional group
amine
hydrazine
SMILES string
Cl.CC(C)(C)NN
InChI
1S/C4H12N2.ClH/c1-4(2,3)6-5;/h6H,5H2,1-3H3;1H
InChI key
DDPWVABNMBRBFI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
tert-Butylhydrazine hydrochloride was used in the synthesis of:
- diphosphinohydrazines
- 5-amino-1-tert-butyl-3-(p-chlorophenyl)-4-cyanopyrazole
- 1,3,4,5-tetrasubstituted pyrazole derivatives
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regioselective synthesis of 1, 3, 4, 5-tetrasubstituted pyrazoles from Baylis-Hillman adducts.
Lee KY, et al.
Tetrahedron Letters, 44(35), 6737-6740 (2003)
One-pot synthesis of tetrasubstituted pyrazoles-Proof of regiochemistry.
Hanefeld U, et al.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1545-1552 (1996)
Alexander N Kornev et al.
Inorganic chemistry, 51(2), 874-881 (2012-01-03)
Reactions of diphosphinohydrazines R-NH-N(PPh(2))(2) (R = tBu (1), Ph(2)P (3)) with some metalation reagents (Co[N(SiMe(3))(2)](2), LiN(SiMe(3))(2), La[N(SiMe(3))(2)](3), nBuLi, MeLi) were performed. Compound 1 was synthesized by the reaction of Ph(2)PCl with tert-butylhydrazine hydrochloride in 83% yield. This compound reveals temperature-dependent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service