All Photos(3)
About This Item
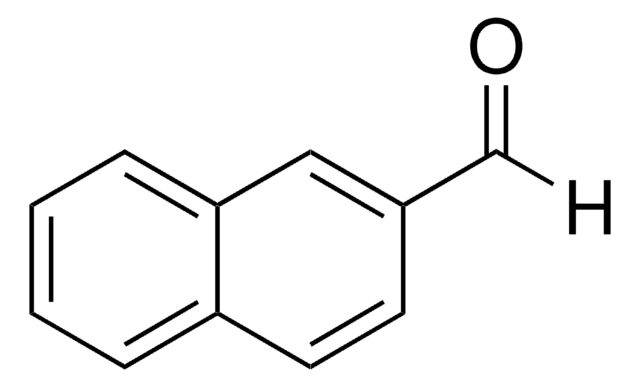
Linear Formula:
C10H7CH2OH
CAS Number:
Molecular Weight:
158.20
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39020101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
79-81 °C (lit.)
functional group
hydroxyl
SMILES string
OCc1ccc2ccccc2c1
InChI
1S/C11H10O/c12-8-9-5-6-10-3-1-2-4-11(10)7-9/h1-7,12H,8H2
InChI key
MFGWMAAZYZSWMY-UHFFFAOYSA-N
Application
2-Naphthalenemethanol was used as marker in the determination of critical micelle concentration of anionic surfactants by capillary electrophoresis. It was used in the synthesis of acetate protected xylosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T P Binder et al.
Molecular pharmacology, 33(4), 477-479 (1988-04-01)
Aryl sulfotransferase (AST) IV catalyzes the 3'-phosphoadenosine 5'-phosphosulfate-dependent sulfation of a variety of benzylic alcohols. Several molecular characteristics of benzylic alcohols were investigated for their ability to influence the catalytic efficiency of a homogeneous preparation of rat hepatic AST IV.
Karin Holmqvist et al.
Bioorganic & medicinal chemistry, 21(11), 3310-3317 (2013-04-23)
Proteoglycans (PGs) are important macromolecules in mammalian cells, consisting of a core protein substituted with carbohydrate chains, known as glycosaminoglycans (GAGs). Simple xylosides carrying hydrophobic aglycons can enter cells and act as primers for GAG chain synthesis, independent of the
Determination of critical micelle concentration of anionic surfactants by capillary electrophoresis using 2-naphthalenemethanol as a marker for micelle formation.
Nakamura H, et al.
Analytical Sciences, 14(2), 379-382 (1998)
Anton Kulikov et al.
The Journal of organic chemistry, 73(19), 7611-7615 (2008-09-11)
Irradiation of alcohols, phenols, and carboxylic acids "caged" with the (3-hydroxy-2-naphthalenyl)methyl group results in fast (k(release) approximately = 10(5) s(-1)) release of the substrates with good quantum (Phi = 0.17-0.26) and chemical (>90%) yields. The initial byproduct of the photoreaction
Fanyu Zhang et al.
Nature communications, 11(1), 1431-1431 (2020-03-20)
The production of 2D metal-organic frameworks (MOFs) with highly exposed active surfaces is of great importance for catalysis. Here we demonstrate the formation of MOF nanosheets by utilizing CO2 as a capping agent to control the oriented growth of MOF. This
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service