180637
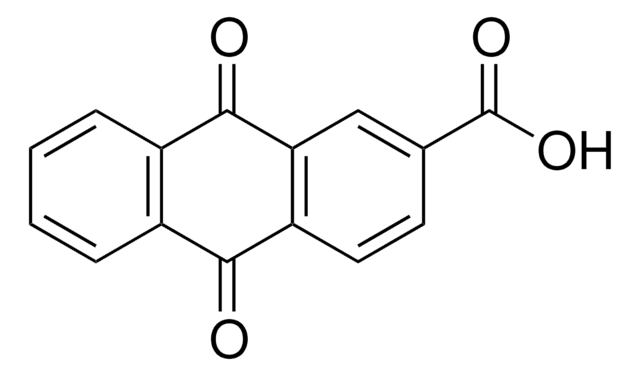
9-Hydroxy-9-fluorenecarboxylic acid
96%
Synonym(s):
Flurenol, 9-Hydroxyfluorene-9-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H10O3
CAS Number:
Molecular Weight:
226.23
Beilstein:
1314711
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
162-166 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OC(=O)C1(O)c2ccccc2-c3ccccc13
InChI
1S/C14H10O3/c15-13(16)14(17)11-7-3-1-5-9(11)10-6-2-4-8-12(10)14/h1-8,17H,(H,15,16)
InChI key
GXAMYUGOODKVRM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
9-Hydroxy-9-fluorenecarboxylic acid was used in the synthesis of a hexameric organooxotin prismane.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vadapalli Chandrasekhar et al.
Journal of the American Chemical Society, 127(33), 11556-11557 (2005-08-18)
A hydroxyl-rich hexameric organooxotin prismane has been prepared by reaction of n-BuSn(O)OH with 9-hydroxy-9-fluorenecarboxylic acid. The supramolecular structure of this cage shows channels with hydrophobic and hydrophilic segments, which selectively entrap guest molecules.
Yusuke Nakajima et al.
Journal of experimental botany, 68(13), 3441-3456 (2017-06-22)
The direction of auxin transport changes in gravistimulated roots, causing auxin accumulation in the lower side of horizontally reoriented roots. This study found that auxin was similarly involved in hydrotropism and gravitropism in rice and pea roots, but hydrotropism in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service