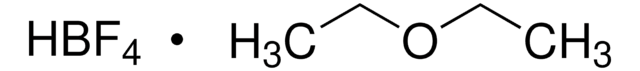175064
Nitrosyl tetrafluoroborate
95%
Synonym(s):
Nitrosonium tetrafluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NOBF4
CAS Number:
Molecular Weight:
116.81
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
reaction suitability
reagent type: oxidant
storage temp.
2-8°C
SMILES string
N#[O+].F[B-](F)(F)F
InChI
1S/BF4.NO/c2-1(3,4)5;1-2/q-1;+1
InChI key
KGCNVGDHOSFKFT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Nitrosyl tetrafluoroborate is an efficient nitrosating and diazotizing agent. It reacts with alcohols and secondary amines to yield alkyl nitrites and nitrosamines, respectively. It reacts with primary amines to yield diazonium tetrafluoroborates. NOBF4 is also a mild oxidant and commonly used for single electron transfer oxidation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Mohr et al.
The Journal of biological chemistry, 274(14), 9427-9430 (1999-03-27)
S-Nitrosylation of protein thiol groups by nitric oxide (NO) is a widely recognized protein modification. In this study we show that nitrosonium tetrafluoroborate (BF4NO), a NO+ donor, modified the thiol groups of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by S-nitrosylation and caused enzyme
Electronically regulated thermally and light-gated electron transfer from anions to naphthalenediimides.
Guha, Samit et al.
Journal of the American Chemical Society, 133(39), 15256-15259 (2011)
S Mohr et al.
FEBS letters, 348(3), 223-227 (1994-07-18)
Previous studies have suggested that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) undergoes covalent modification of an active site thiol by a NO.-induced [32P]NAD(+)-dependent mechanism. However, the efficacy of GAPDH modification induced by various NO donors was found to be independent of spontaneous rates
J Li et al.
Biochemical and biophysical research communications, 240(2), 419-424 (1997-12-06)
The caspases are a family of at least 10 human cysteine proteases that participate in cytokine maturation and in apoptotic signal transduction and execution mechanisms. Peptidic inhibitors of these enzymes are capable of blocking cytokine maturation and apoptosis, demonstrating their
C Würth et al.
Nanoscale, 9(12), 4283-4294 (2017-03-16)
The rational design of brighter upconversion nanoparticles (UCNPs) requires a better understanding of the radiationless deactivation pathways in these materials. Here, we demonstrate the potential of excitation power density (P)-dependent studies of upconversion (UC) luminescence intensities, slope factors, and absolute
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service