171824
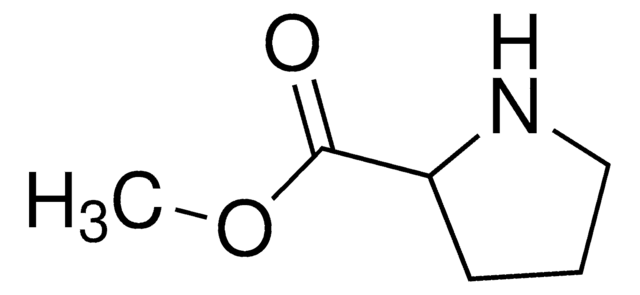
DL-Proline
99%, for peptide synthesis, ReagentPlus®
Synonym(s):
(±)-Pyrrolidine-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H9NO2
CAS Number:
Molecular Weight:
115.13
Beilstein:
80812
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
DL-Proline, ReagentPlus®, 99%
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
208 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)C1CCCN1
InChI
1S/C5H9NO2/c7-5(8)4-2-1-3-6-4/h4,6H,1-3H2,(H,7,8)
InChI key
ONIBWKKTOPOVIA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
DL-Proline, also known as 2-pyrrolidinylcarboxylic acid, is an amino acid commonly used in solution-phase peptide synthesis due to its ability to act as a turn inducer resulting from its restricted phi( ϕ) angle.
Application
DL-Proline can be is used in the preparation of cis-aminoproline-alkene (cis-Apa)linker, which is further used as an intermediate compound for the synthesis of cyclic pseudopeptides via solution phase peptide synthesis.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
cis-Apa: A practical linker for the microwave-assisted preparation of cyclic pseudopeptides via RCM cyclative cleavage
A Baron
The Journal of Organic Chemistry, 76, 766-772 (2011)
Recent Progress in Solid-Phase Total Synthesis of Naturally Occurring Small Peptides
H Yan
Advanced Synthesis & Catalysis, 364, 1934-1961 (2022)
Antoinette Bugyei-Twum et al.
Cardiovascular diabetology, 13, 89-89 (2014-06-03)
Despite advances in the treatment of heart failure, mortality remains high, particularly in individuals with diabetes. Activated transforming growth factor beta (TGF-β) contributes to the pathogenesis of the fibrotic interstitium observed in diabetic cardiomyopathy. We hypothesized that high glucose enhances
Nina Maeshima et al.
The Journal of biological chemistry, 290(21), 13440-13453 (2015-04-04)
Lipid A in LPS activates innate immunity through the Toll-like receptor 4 (TLR4)-MD-2 complex on host cells. Variation in lipid A has significant consequences for TLR4 activation and thus may be a means by which Gram-negative bacteria modulate host immunity.
Souhir Marsit et al.
Molecular biology and evolution, 32(7), 1695-1707 (2015-03-10)
Although an increasing number of horizontal gene transfers have been reported in eukaryotes, experimental evidence for their adaptive value is lacking. Here, we report the recent transfer of a 158-kb genomic region between Torulaspora microellipsoides and Saccharomyces cerevisiae wine yeasts
Chromatograms
application for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service