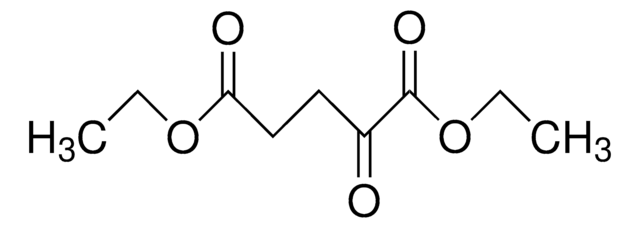
171263
Diethyl oxalacetate sodium salt
95%
Synonym(s):
Diethyl oxalacetate, Diethyl oxaloacetate, Diethyl oxosuccinate sodium salt, Oxalacetic acid diethyl ester sodium salt, Sodium diethyl oxalacetate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C2H5OCOC(ONa)=CHCOOC2H5
CAS Number:
Molecular Weight:
210.16
Beilstein:
4279609
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
188-190 °C (lit.)
functional group
ester
SMILES string
[Na+].CCOC(=O)\C=C(/[O-])C(=O)OCC
InChI
1S/C8H12O5.Na/c1-3-12-7(10)5-6(9)8(11)13-4-2;/h5,9H,3-4H2,1-2H3;/q;+1/p-1/b6-5-;
InChI key
UJZUICGIJODKOS-YSMBQZINSA-M
Application
Diethyl oxalacetate sodium salt can be used as a reagent to synthesize:
- Spiro[indoline-3,4′-pyrano[2,3-c]pyrazole derivatives via a one-pot reaction with isatins, malononitrile, and hydrazine hydrate.
- 2,3-dioxo-4-carboxy-5-substituted pyrrolidines by reacting with various aldehydes and primary amines.
- 2-nitro-4-fluoro triazole diester by treating with 2-nitro-4-fluoro-phenylazide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New 1, 2, 3-triazolo [1, 5-a] quinoxalines: synthesis and binding to benzodiazepine and adenosine receptors. II
Biagi G, et al.
European Journal of Medicinal Chemistry, 37(7), 565-571 (2002)
Synthesis of novel 3, 4-fused pyrazolidinone ?-lactam bicyclic moieties from 2, 3-dioxo-4-carboxy-5-(substituted) pyrrolidines
Rashid FNAA, et al.
Organic Communications, 12(3), 121-131 (2019)
Diethyl oxalacetate sodium salt as a reagent to obtain functionalized spiro [indoline-3, 4?-pyrano [2, 3-c] pyrazoles]
Gein VL, et al.
Tetrahedron Letters, 58(2), 134-136 (2017)
PYRUVATE CARBOXYLASE. I. NATURE OF THE REACTION.
M F UTTER et al.
The Journal of biological chemistry, 238, 2603-2608 (1963-08-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service