156957
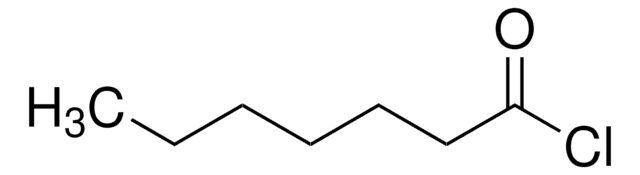
Hexanoyl chloride
97%
Synonym(s):
Caproyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4COCl
CAS Number:
Molecular Weight:
134.60
Beilstein:
506332
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.426 (lit.)
bp
150-153 °C (lit.)
solubility
chloroform: soluble(lit.)
diethyl ether: soluble(lit.)
density
0.963 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
CCCCCC(Cl)=O
InChI
1S/C6H11ClO/c1-2-3-4-5-6(7)8/h2-5H2,1H3
InChI key
YWGHUJQYGPDNKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Hexanoyl chloride was used in:
- total synthesis of (±)-7-butyl-6,8-dihydroxy-3-pentyl-3,4-dihydroisochromen-1-one
- asymmetric synthesis of 14-methyl-1-octadecene, the sex pheromone of the peach leafminer moth
- preparation of hexanoyl-coated nanofibers dispersible in several organic solvents
- total synthesis of natural isocarbostyril ruprechstyril (3-n-pentyl-6-methoxy-8-hydroxy-1(2H)-isoquinolinone), isolated from Ruprechtia tangarana
- synthesis of 5-chloro-8-hydroxy-6-methoxy-3-pentylisocoumarin, the 5-chloro analog of naturally occurring 7-chloro-8-hydroxy-6-methoxy-3-pentylisocoumarin, isolated from Tessmannia densiflora
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aamer Saeed
Journal of Asian natural products research, 13(6), 505-511 (2011-05-31)
The synthesis of title isocoumarin, the 5-chloro analog of naturally occurring 7-chloro-8-hydroxy-6-methoxy-3-pentylisocoumarin, isolated from Tessmannia densiflora is described. Chlorination of ethyl 2-(2-ethoxy-2-oxoethyl)-4,6-dimethoxybenzoate (2) afforded 3-chloro ester (3) followed by hydrolysis to furnish the 2-(carboxymethyl)-3-chloro-4,6-dimethoxybenzoic acid (4) that was converted to
Tao Zhang et al.
Molecules (Basel, Switzerland), 18(5), 5201-5208 (2013-05-09)
An asymmetric synthesis of 14-methyl-1-octadecene, the sex pheromone of the peach leafminer moth has been achieved. The target molecule was synthesized in six linear steps and in 30.3% overall yield from commercially available hexanoyl chloride, (S)-4-benzyloxazolidin-2-one and 1,9-nonanediol. The hexanoyl
Aamer Saeed
Natural product research, 27(13), 1153-1158 (2012-08-14)
A short total synthesis of natural isocarbostyril ruprechstyril (3-n-pentyl-6-methoxy-8-hydroxy-1(2H)-isoquinolinone) isolated from Ruprechtia tangarana is reported. 6,8-Dimethoxy-3-pentylisocoumarin obtained by condensation of 3,5-dimethoxyhomophthalic anhydride with hexanoyl chloride was smoothly converted to O-methylruprechstyril by refluxing with methanamide. Regioselective demethylation of the latter using
Aamer Saeed et al.
Journal of Asian natural products research, 15(10), 1112-1122 (2013-07-23)
A new total synthesis of ( ± )-7-butyl-6,8-dihydroxy-3-pentyl-3,4-dihydroisochromen-1-one, isolated as R-enantiomer from Geotrichum sp., has been described. Reaction of 4-butyl-3,5-dimethoxyhomophthalic anhydride with hexanoyl chloride in the presence of 1,1,3,3-tetramethyl guanidine and triethyl amine afforded 7-butyl-6,8-dimethoxy-3-pentylisochromen-1-one, which was converted into corresponding 3,4-dihydroisochromen-1-one by
One-step dispersion of cellulose nanofibers by mechanochemical esterification in an organic solvent.
Pei Huang et al.
ChemSusChem, 5(12), 2319-2322 (2012-11-28)
Got a crush: Native cellulose can be dispersed as nanofibers in organic solvents by ball milling with esterification agents. Milling with hexanoyl chloride/DMF gives hexanoyl-coated nanofibers dispersible in several organic solvents. Milling with succinic anhydride/DMSO results water-dispersible nanofibers. The results
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service