152625
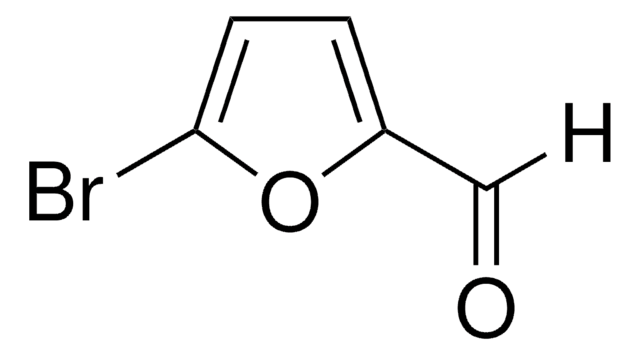
5-Bromo-2-thiophenecarboxaldehyde
95%
Synonym(s):
5-Bromothenaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
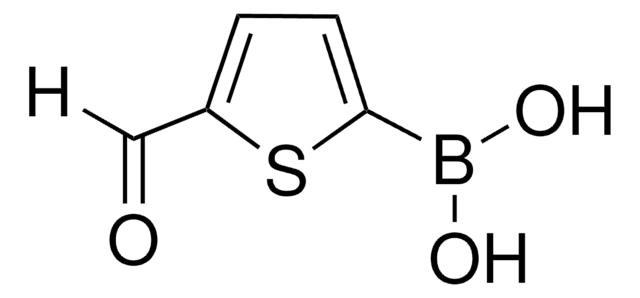
Empirical Formula (Hill Notation):
C5H3BrOS
CAS Number:
Molecular Weight:
191.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.637 (lit.)
bp
105-107 °C/11 mmHg (lit.)
density
1.607 g/mL at 25 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccc(Br)s1
InChI
1S/C5H3BrOS/c6-5-2-1-4(3-7)8-5/h1-3H
InChI key
GFBVUFQNHLUCPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Bromo-2-thiophenecarboxaldehyde was used to prepare 5-[18F]fluoro-2-2-thiophene carboxaldehyde.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M R Kilbourn
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 16(7), 681-686 (1989-01-01)
Possible applications of thiophenes in radiopharmaceutical chemistry have been explored. Thiophene for benzene ring substitution was applied to the synthesis of thienyl-[18F]GBR 13119, an analog of the potent and selective dopamine uptake inhibitor [18F]GBR 13119. In vivo regional brain distribution
Ismail Warad et al.
Molecules (Basel, Switzerland), 25(9) (2020-05-15)
Three new tetradentate NNNS Schiff bases (L1-L3) derived from 2-(piperidin-4-yl)ethanamine were prepared in high yields. UV-Visible and FTIR spectroscopy were used to monitor the dehydration reaction between 2-(piperidin-4-yl)ethanamine and the corresponding aldehydes. Structures of the derived Schiff bases were deduced
Hyojung Cha et al.
ACS applied materials & interfaces, 6(18), 15774-15782 (2014-08-26)
The molecular packing structures of two conjugated polymers based on alkoxy naphthalene, one with cyano-substituents and one without, have been investigated to determine the effects of electron-withdrawing cyano-groups on the performance of bulk-heterojunction solar cells. The substituted cyano-groups facilitate the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service