All Photos(1)
About This Item
Empirical Formula (Hill Notation):
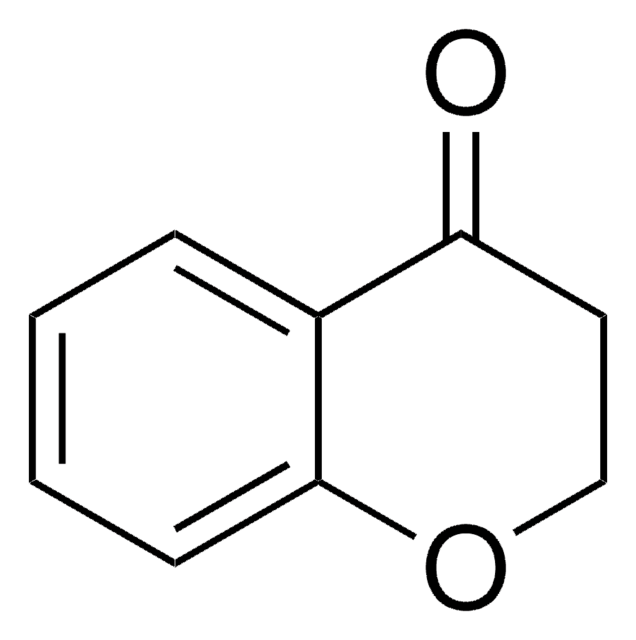
C9H8O
CAS Number:
Molecular Weight:
132.16
Beilstein:
636550
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
impurities
≤2.0% water
mp
51-54 °C (lit.)
functional group
ketone
storage temp.
2-8°C
SMILES string
O=C1Cc2ccccc2C1
InChI
1S/C9H8O/c10-9-5-7-3-1-2-4-8(7)6-9/h1-4H,5-6H2
InChI key
UMJJFEIKYGFCAT-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Indanone undergoes TiCl4-Mg mediated coupling with CHBr3 to yield dibromomethyl carbinol. It reacts with 5,5-dimethyl-3-pyrazolidinone to yield 5,5-dimethyl-2-(1H-indenyl-2)-3-pyrazolidinone. 2-Indanone on photolysis by 266-nm one-photon excitation yields o-xylylene.
Application
2-Indanone was used as starting reagent in the synthesis of indene-fused porphyrins.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
212.0 °F - closed cup
Flash Point(C)
100 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tu-Hsin Yan et al.
Organic letters, 15(22), 5802-5805 (2013-11-07)
TiCl4-Mg can mediate addition of CHBr3 to a variety of aldehydes and ketones to form dibromomethyl carbinols and also be used to effect CBr3 transfer to carbonyl groups to form tribromomethyl carbinols. The successful application of TiCl4-Mg-promoted coupling of CHBr3
Spectroscopic studies on photochemical formation of o-xylylene in solution.
Fujiwara M, et al.
The Journal of Physical Chemistry A, 101(27), 4912-4915 (1997)
Timothy D Lash et al.
The Journal of organic chemistry, 76(13), 5335-5345 (2011-05-24)
Indene-fused porphyrins have been synthesized starting from 2-indanone. Knorr-type reaction of oximes derived from benzyl or tert-butyl acetoacetate with 2-indanone and zinc dust in propionic acid gave good yields of indenopyrroles. Treatment with N-chlorosuccinimide then gave 8-chloro derivatives, and these
Laura E Korhonen et al.
Journal of medicinal chemistry, 48(11), 3808-3815 (2005-05-27)
The purpose of this study was to determine the cytochrome P450 1A2 (CYP1A2) inhibition potencies of structurally diverse compounds to create a comprehensive three-dimensional quantitative structure-activity relationship (3D-QSAR) model of CYP1A2 inhibitors and to use this model to predict the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service