All Photos(1)
About This Item
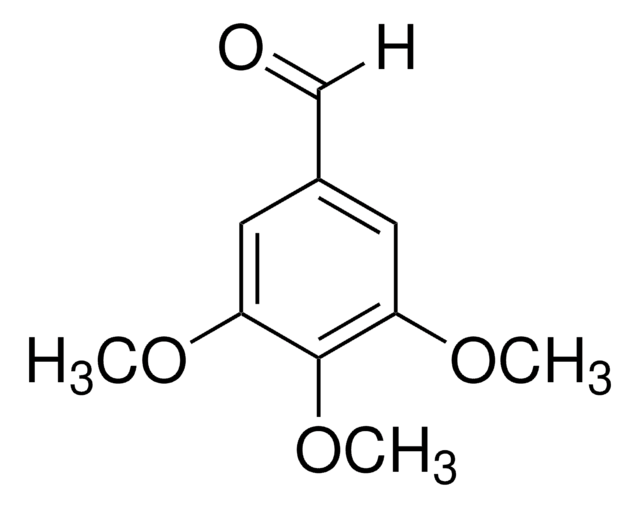
Linear Formula:
(CH3O)3C6H2CHO
CAS Number:
Molecular Weight:
196.20
Beilstein:
1956051
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
115-120 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1c(OC)cc(OC)cc1OC
InChI
1S/C10H12O4/c1-12-7-4-9(13-2)8(6-11)10(5-7)14-3/h4-6H,1-3H3
InChI key
CRBZVDLXAIFERF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4,6-Trimethoxybenzaldehyde exhibits significant anti-candida activity.
Application
2,4,6-Trimethoxybenzaldehyde was used in the preparation and characterization of three RNA-specifc fluorescent probes (E36, E144 and F22) and their use in live cell imaging. It was used as starting reagent for the regioselective synthesis of new (+/-)-8-alkyl-5,7-dihydroxy-4-(4-hydroxyphenyl)-3,4-dihydrocoumarins.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asma Alshamari et al.
Molecules (Basel, Switzerland), 25(18) (2020-09-24)
A series of derivatives of trans-3-(2,4,6-trimethoxyphenyl)4,5-dihydroisoxazolo-4,5-bis[carbonyl-(4'phenyl)thiosemicarbazide (9) and of trans-3-(2,4,6-trimethoxyphenyl)-4,5-dihydro isoxazolo-4,5-bis(aroylcarbohydrazide) (10a-c) were synthesized from trans-3-(2,4,6-trimethoxyphenyl)-4,5-dihydro-4,5-bis(hydrazenocarbonyl)isoxazole (8). The structures of the compounds were elucidated by both elemental and spectral (IR, NMR, and MS) analysis. Compound 9 shows activity against some
Sandeep B Rajput et al.
Chinese medicine, 8(1), 18-18 (2013-09-10)
Asaronaldehyde (2, 4, 5-trimethoxybeznaldehyde) is an active component of Acorus gramineus rhizome. This study aims to evaluate the anti-Candida efficacy of asaronaldehyde and its three structural isomers, namely, 2, 3, 4-trimethoxybenzaldehyde, 3, 4, 5-trimethoxybenzaldehyde, and 2, 4, 6- trimethoxybenzaldehyde. Susceptibility
Lili Du et al.
Theranostics, 7(14), 3432-3445 (2017-09-16)
Small interfering RNA (siRNA) therapies have been hampered by lack of delivery systems in the past decades. Nowadays, a few promising vehicles for siRNA delivery have been developed and it is gradually revealed that enhancing siRNA release from endosomes into
Fatemeh Oroojalian et al.
Journal of controlled release : official journal of the Controlled Release Society, 288, 45-61 (2018-09-02)
In the current study, thermoresponsive poly(N-isopropylacrylamide)-doxorubicin (PNIPAM-DOX) hydrogel was synthesized and loaded into pH-responsive poly ethylene glycol)-2,4,6- trimethoxy benzylidene pentaerythritol carbonate (PEG-PTMBPEC) polymersomes in order to fabricate a smart thermo-pH stimuli responsive drug delivery system. Thermo-pH responsive polymersomal formulation of
Frederik Roelens et al.
European journal of medicinal chemistry, 40(10), 1042-1051 (2005-06-14)
Nine new (+/-)-8-alkyl-5,7-dihydroxy-4-(4-hydroxyphenyl)-3,4-dihydrocoumarins have been synthesized from 2,4,6-trimethoxybenzaldehyde via a short, efficient, and regioselective pathway, together with the unsubstituted analogue (+/-)-5,7-dihydroxy-4-(4-hydroxyphenyl)-3,4-dihydrocoumarin. The compounds were tested for estrogenic activity using a yeast-based estrogen screen. Weak estrogenicity was determined for seven members
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service