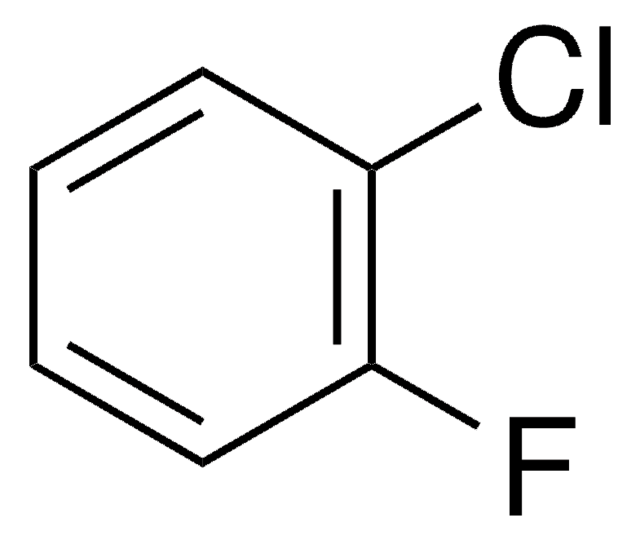
126152
1,2-Difluorobenzene
98%
Synonym(s):
o-Difluorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4F2
CAS Number:
Molecular Weight:
114.09
Beilstein:
1905113
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.443 (lit.)
bp
92 °C (lit.)
mp
−34 °C (lit.)
density
1.158 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccccc1F
InChI
1S/C6H4F2/c7-5-3-1-2-4-6(5)8/h1-4H
InChI key
GOYDNIKZWGIXJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,2-Difluorobenzene undergoes defluorination under very mild conditions by H2 in the presence of NaOAc over rhodium pyridylphosphine and bipyridyl complexes tethered on a silica-supported palladium catalyst.
Application
1,2-Difluorobenzene(1,2-DFB) has been used to study the mechanism of dissociation of o-, m- and p-difluorobenzene ions by threshold photoelectron photoion coincidence spectroscopy. It has been used to study the room temperature adsorption of 1,2-DFB, 1,2-dichlorobenzene and 1,2-dibromobenzene on Si(100)2x1 by X-ray photoelectron spectroscopy and temperature programmed desorption. It was used as solvent in electrochemical studies on transition metal complexes.
Solvent useful for electrochemical studies on transition metal complexes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
33.8 °F - closed cup
Flash Point(C)
1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anne-Marie Boulanger et al.
Journal of the American Society for Mass Spectrometry, 20(1), 20-24 (2008-10-18)
Threshold photoelectron photoion coincidence (TPEPICO) experiments have shown that o-, m-, and p-difluorobenzene ions dissociate via a common, ring-opened intermediate and not via ionized p-difluorobenzene. Rice-Ramsperger-Kassel-Marcus (RRKM) modeling of the experimental breakdown curves yields activation energies for the initial isomerization
Hydrodefluorination of fluorobenzene and 1, 2-difluorobenzene under mild conditions over rhodium pyridylphosphine and bipyridyl complexes tethered on a silica-supported palladium catalyst.
Yang H, et al.
Organometallics, 18(12), 2285-2287 (1999)
Inorganic Chemistry, 28, 3923-3923 (1989)
Competition between associative and dissociative adsorption of 1, 2-dihalogenated benzenes on Si (100) 2? 1: Formation of dihalocyclohexadiene, halophenyl and phenylene adstructures.
Zhou XJ and Leung KT.
Surface Science, 600(16), 3285-3296 (2006)
Adrian Romero-Flores et al.
Chemosphere, 186, 151-159 (2017-08-05)
Electronic noses have been widely used in the food industry to monitor process performance and quality control, but use in wastewater and biosolids treatment has not been fully explored. Therefore, we examined the feasibility of an electronic nose to discriminate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service