116017
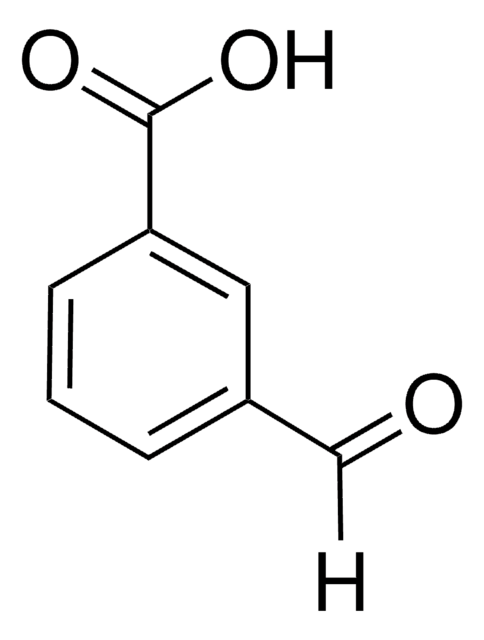
2-Carboxybenzaldehyde
97%
Synonym(s):
Phthalaldehydic acid, 2-Formylbenzoic acid, 3-Hydroxyphthalide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H6O3
CAS Number:
Molecular Weight:
150.13
Beilstein:
742381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
94-96 °C (lit.)
functional group
aldehyde
carboxylic acid
SMILES string
OC(=O)c1ccccc1C=O
InChI
1S/C8H6O3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H,(H,10,11)
InChI key
DYNFCHNNOHNJFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Carboxybenzaldehyde is an aldehydic acid that can be used to synthesize functionalized diazaphospholanes.
Application
2-Carboxybenzaldehyde is a metabolite of the biodegradation of luoranthene using two pure bacterial strains, Pasteurella sp. IFA (B-2) and Mycobacterium sp. PYR-1 (AM). It has been used to synthesize a series of N-substituted isoindolinones by reductive C-N coupling and intramolecular amidation with amines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solid-phase synthesis of chiral 3, 4-diazaphospholanes and their application to catalytic asymmetric allylic alkylation
Landis CR and Clark TP
Proceedings of the National Academy of Sciences, 101, 5428-5432 (2004)
E Sepic et al.
Journal of applied microbiology, 85(4), 746-754 (1998-11-13)
The findings from a biodegradability study of fluoranthene using two pure bacterial strains, Pasteurella sp. IFA (B-2) and Mycobacterium sp. PYR-1 (AM) are reported. Of total fluoranthene, 24% (B-2) and 46% (AM) was biodegraded in an aqueous medium during 14
Linyan Shi et al.
Organic letters, 14(7), 1876-1879 (2012-03-17)
A series of N-substituted isoindolinones have been successfully synthesized through the reductive C-N coupling and intramolecular amidation of 2-carboxybenzaldehyde and amines. This one-pot synthesis gives excellent yields using ultrathin Pt nanowires as catalysts under 1 bar of hydrogen. These unsupported
Yuling Bao et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 129, 110377-110377 (2020-06-20)
There are plenty of evidences to show that combining of chemotherapy and immunotherapy should be a very potent anti-cancer therapeutic method. In this research, we evaluated the anti-tumor activity of doxorubicin prodrug combined with erythrocyte membrane-enveloped polymer nano-vaccine against tumor
Anna Muratova et al.
Biodegradation, 25(6), 787-795 (2014-07-24)
The biodegradation of the polycyclic aromatic hydrocarbon phenantherene by the rhizobacterial strain Ensifer meliloti P221, isolated from the root zone of plant grown in PAH-contaminated soil was studied. Bacterial growth and phenanthrene degradation under the influence of root-exuded organic acids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service