110094
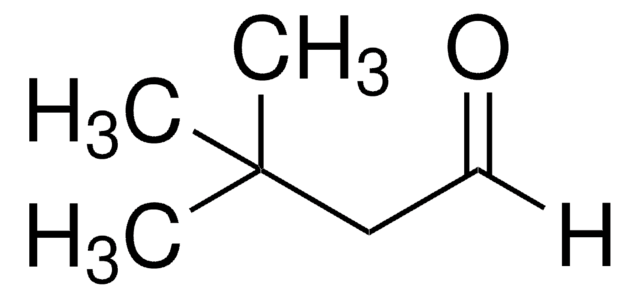
2-Ethylbutyraldehyde
≥92%
Synonym(s):
2-Ethylbutanal
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C2H5)2CHCHO
CAS Number:
Molecular Weight:
100.16
Beilstein:
1209330
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥92%
refractive index
n20/D 1.402 (lit.)
bp
117 °C (lit.)
density
0.814 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
CCC(CC)C=O
InChI
1S/C6H12O/c1-3-6(4-2)5-7/h5-6H,3-4H2,1-2H3
InChI key
UNNGUFMVYQJGTD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Ethylbutyraldehyde reacts with cyclohexanecarbaldehyde to form homoallylic alcohols.
Application
2-Ethylbutyraldehyde has been used in the preparation of aldoximes using aqueous hydroxylamine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Controlling Selectivity in the Consecutive Reaction Network of Aldoxime Hydrogenation to Primary Amines.
Gebauer-Henke E, et al. et al.
Synthesis, 102 null
Highly diastereoselective reactions using masked allylic zinc reagents.
Jones P and Knochel P.
Chemical Communications (Cambridge, England), 21, 2407-2408 (1998)
Noa T Sorbara et al.
Organic & biomolecular chemistry, 17(37), 8618-8627 (2019-09-19)
A rational approach that may be applied to a broad class of enzyme-catalyzed reactions to design enzyme inhibitors affords a powerful strategy, facilitating the development of drugs and/or molecular probes of enzyme mechanisms. A strategy for the development of substrate-product
Yusuke Yamamoto et al.
The Journal of toxicological sciences, 44(9), 585-600 (2019-09-03)
Amino acid derivative reactivity assay (ADRA) has previously been developed as an alternative method to direct peptide reactivity assay (DPRA) to evaluate key event 1 in skin sensitization mechanisms. However, when using alternative methods for skin sensitization, integrated approaches to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![2-Azabicyclo[2.2.1]hept-5-en-3-one 98%](/deepweb/assets/sigmaaldrich/product/structures/155/017/1874f631-2345-407a-83c6-4ef0fa3f35a3/640/1874f631-2345-407a-83c6-4ef0fa3f35a3.png)
