All Photos(1)
About This Item
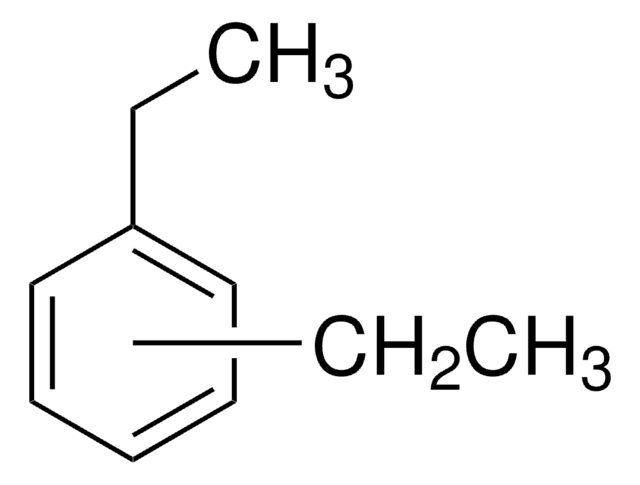
Linear Formula:
C6H4(C2H5)2
CAS Number:
Molecular Weight:
134.22
Beilstein:
1904392
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
92%
form
liquid
autoignition temp.
743 °F
refractive index
n20/D 1.502 (lit.)
bp
183 °C (lit.)
mp
−31 °C (lit.)
density
0.88 g/mL at 25 °C (lit.)
SMILES string
CCc1ccccc1CC
InChI
1S/C10H14/c1-3-9-7-5-6-8-10(9)4-2/h5-8H,3-4H2,1-2H3
InChI key
KVNYFPKFSJIPBJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
131.0 °F - closed cup
Flash Point(C)
55 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
I Linhart et al.
Xenobiotica; the fate of foreign compounds in biological systems, 26(12), 1263-1272 (1996-12-01)
1. Biotransformation of 1,2-diethenylbenzene (1) in rat was studied. Five urinary metabolites were isolated by extraction of acid hydrolysed urine and identified by nmr and mass spectroscopy, namely, 1-(2-ethenylphenyl)ethane-1,2-diol (2) 2-ethenylmandelic acid (3), 2-ethenylphenylglyoxylic acid (4), 2-ethenylphenylacetylglycine (5) N-acetyl-S-[1-(2-ethenylphenyl)-2-hydroxyethyl]cysteine (6)
J P Payan et al.
Drug metabolism and disposition: the biological fate of chemicals, 29(6), 868-876 (2001-05-17)
In a previous study, it was shown that the neurotoxic compound 1,2-diethylbenzene (1,2-DEB) is mainly hydroxylated in the alkyl chain to give 1-(2'-ethylphenyl)ethanol (1,2-EPE) and excreted in urine of rats as two glucuronide compounds (GA1 and GA2). Some findings have
Jean-Paul Payan et al.
Archives of toxicology, 82(9), 591-600 (2008-02-07)
The bio-distribution of the neurotoxic 1,2-diethylbenzene (1,2-DEB) was studied in male Sprague-Dawley rats after intravenous administration of [(14)C] 1,2-DEB (1 mg kg(-1)). The highest concentrations of [(14)C] non-volatile metabolites, determined by whole-body auto-radiography, were in the nasal cavity, ethmoid turbinates
J P Payan et al.
Drug metabolism and disposition: the biological fate of chemicals, 27(12), 1470-1478 (1999-11-26)
The excretion and metabolism of neurotoxic 1,2-diethylbenzene (1, 2-DEB) was studied in male Sprague-Dawley rats after i.v. (1 mg/kg) or oral (1 or 100 mg/kg) administration of 1,2-diethyl[U-(14)C]benzene ([(14)C]1,2-DEB). Whatever the treatment, radioactivity was mainly excreted in urine (65-76% of
Karla D Thrall et al.
Journal of toxicology and environmental health. Part A, 70(1), 67-72 (2006-12-13)
Diethylbenzene (DEB) is a moderately volatile, colorless liquid found in gasoline, kerosene, and fuel oils. Exposure to DEB has been shown to produce peripheral neuropathy in rats, and the ortho isomer of DEB (1,2-DEB) is generally believed to be the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service